Glossary
Curie Temperature
The temperature at which a transition between the ferromagnetic and paramagnetic phases occurs.
In physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which magnetic materials lose their ferromagnetic properties, to be replaced by paramagnetism. The Curie temperature is named after Pierre Curie (1859-1906), who showed that magnetism was lost at a critical temperature.
The ferromagnetic elements and alloys with their Curie temperatures – e.g.:
| Material | Curie Temperature |
|---|---|
| Fe | 770°C |
| Co | 1115°C |
| Ni | 354°C |
| Gd | 19°C |
| AlNiCo | 850°C |
| Ferrite | 450°C |
| Sm Cobalt | 750 - 825°C |
| Nd-Fe-B | 310 - 340°C |

Do you have any questions?
Suitable products for your measurement
Application Examples
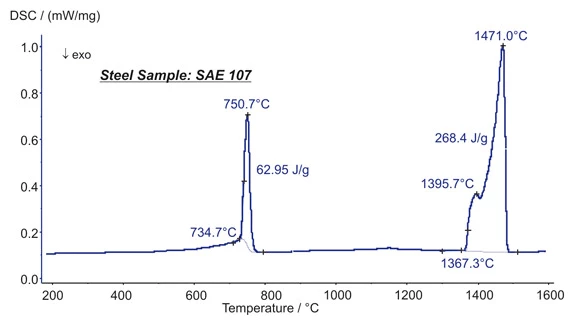
Presented here are the transformation energetics of a steel (SAE 107). At 751°C, two Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transitions overlap each other. The increase in the heat flow rate up to 735°C is due to the Curie transition (change in magnetic properties). A change in the crystal structure (bcc to fcc structure) is causing the major peak. The structural change is connected with an enthalpy change of 63 J/g. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting was seen at 1367°C (extrapolated onset) and occurred in two steps (peaks at 1395°C and 1471°C). The heat of fusion was 268 J/g. (measurement with the DSC 404 F1 Pegasus®®)
Iron
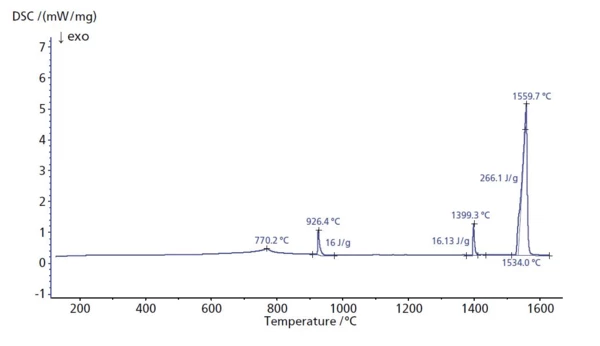
The specific heat flow rate of iron was measured between room temperature and 1620°C. The peak at 770°C is due to a change in magnetic properties of the material (the Curie transition). At peak temperatures of 926°C and 1399°C, two changes in the crystal structure occurred. Most likely due to impurities in the material, these temperatures are slightly shifted compared to the literature values for pure iron [1]. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state.
The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting occurred at 1534°C (extrapolated onset). The heat of fusion was 266 J/g. This is less than a 1.5% deviation from typical literature values for pure iron.
[1] Das Techniker Handbuch, Grundlagen und Anwendungen der Maschinenbau-Technik, 15. Auflage, Herausgeber Alfred Böge, Vieweg Verlag, 1999