POLYMERS
Glass Fiber Filled Epoxy — Curing
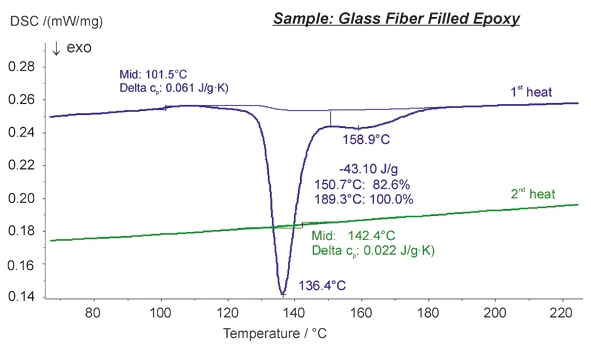
Analysis and optimization of the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing process of epoxy resins can be easily carried out using differential scanning calorimetry.
Presented here is a measurement on a glass fiber filled epoxy measured in the DSC 200 F3 Maia®. The two-step ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal cross-linking reaction slightly above the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition (at 101.5°C) is clearly visible during the first heating of the sample. After a controlled cooling at 5 K/min, the sample was heated a second time. Only a weak Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is visible at a higher temperature (142.4°C) compared to the first heating cycle.