
25.04.2022 by Dr. Elena Moukhina, Dr. Natalie Rudolph, Dr. Stefan Schmölzer
3D Printing: Crystallization Kinetics of Polyamide 12 during Selective Laser Sintering
Powder Bed Fusion (PBF), also called Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering (SLS), is the layer-by-layer construction technology of 3D objects, where a laser beam selectively traces over a predefined area on the powder layer. One of the most widely used materials is PA12.
Powder Bed Fusion (PBF), also called Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering (SLS), is the layer-by-layer construction technology of 3D objects, where a laser beam selectively traces over a predefined area on the powder layer. The laser beam causes the powder to melt and upon application of the next layer of (colder) powder, it might initiate CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization. This process is repeated until the whole part has been created. A full description of the process can be found in our blog article on SLS [2].
One of the most widely used materials is PA12, but modifications or other materials with improved or different properties are constantly developed.
Before working with a new material, it is very important to know the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior of the new material in order to find the optimal temperatures for the SLS process. These temperatures are one of the main parameters of SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering process, influencing the speed of SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering as well as the final product quality. The common trial-and-error approach is very time consuming and therefore expensive. In contrast, qualification of a new material can be undertaken much faster using the Kinetics Neo software for kinetic modelling of CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization rate based on Differential Scanning Calorimetry (DSC) data, followed by a simulation of the process for different temperature profiles.
Firstly, the experimental DSC measurements are conducted, followed by kinetics analysis on this data to create the kinetic model. Finally, the model is used when simulating different processing temperature scenarios in order to find the most optimal one.
Experimental
DSC enables the determination of melting and crystallization temperatures during heating and cooling. These temperatures define the process window of working temperatures for SLS technology [1]. However, these temperatures depend on the heating and cooling rates because both processes are time-dependent. For lower heating and cooling rates, the process window will be reduced. This requires IsothermalTests at controlled and constant temperature are called isothermal.isothermal measurements [2].
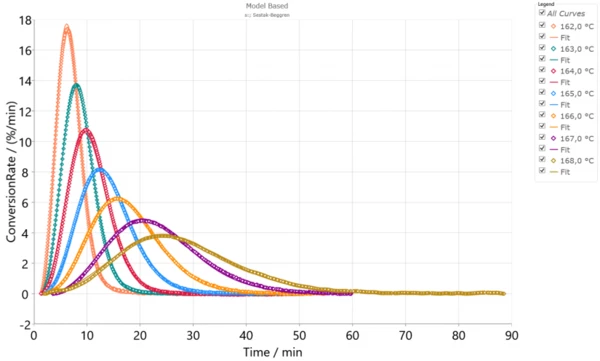
IsothermalTests at controlled and constant temperature are called isothermal.Isothermal measurements provide information about the rate of IsothermalTests at controlled and constant temperature are called isothermal.isothermal CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization at different temperatures. This CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization rate depends on the degree of supercooling of a material. For example, the lower the temperature, the higher the degree of supercooling and therefore the higher theCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization rate. This dependence is notable on the experimental measurements for PA12, performed with the DSC 214 Polyma (Fig.1). The experiments were carried out on PA12 samples with a mass of approximately 5 mg in an aluminum pan (Concavus® Al) with a closed lid under nitrogen. The IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment shown here follows a fast cooling ramp from temperatures above the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature.
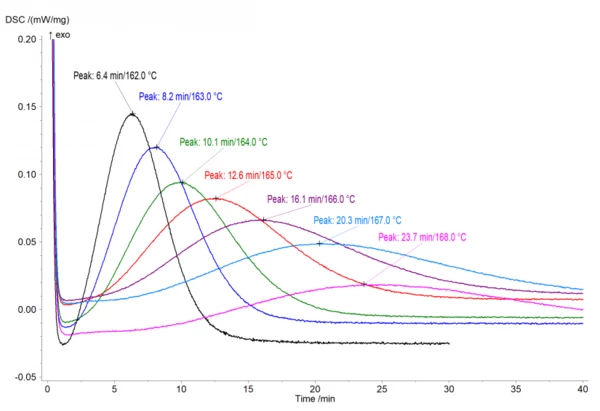
Kinetic Analysis
The kinetic analysis of the DSCisothermal crystallization measurements, at different temperatures, was performed using the NETZSCH Kinetics Neo software. It provided one kinetic model depending on time and temperature, which can describe all experimental curves under different temperatures. This model calculates theCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization rate by the kinetic equation:

In IsothermalTests at controlled and constant temperature are called isothermal.isothermal analysis ofCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization, the first dependence is typically represented by the Avrami equation, which representsCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization nucleation rate.
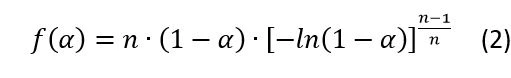
The extended version of the Avrami equation (4, see end of article) is the Sestak-Berggren equation (5, see end of article). This extended equation is used in the current analysis because it provides a better fit for the experimental data
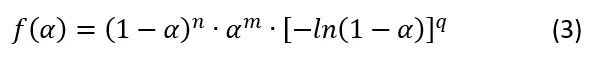
The dependence K(T) in Eq(1) is a formal Arrhenius equation as the decreasing function of temperature with pre-exponent A and apparent activation energy E:
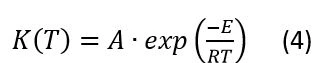
This kinetic model (Eq1) presents the dependence of the current crystallization rate on the temperature and current degree of crystallization.
The equations contain unknown parameters, which are found by the software in order to determine the best fit for the experimental curves.
If this simulation is performed for temperature conditions of the IsothermalTests at controlled and constant temperature are called isothermal.isothermal experiments with optimal parameters, there will be very good agreement between the experiment and simulation with R2=0.998. In Figure 2, the points represent the experimental data and the solid lines, the simulation, according to Eqs. (1,3,4).
Simulations
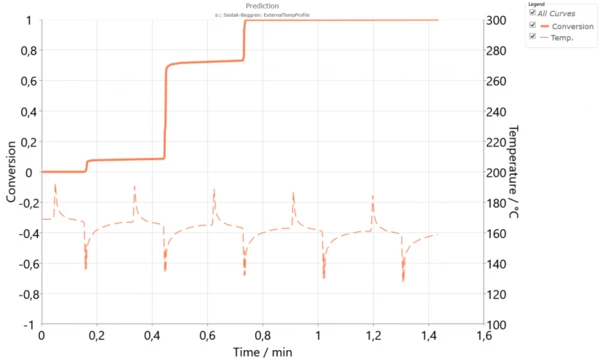
This single model now works for different temperatures. Therefore, it can be used for simulation of crystallization in the SLS process. The temperature profile of the powder surface can be measured over the duration of multiple cycles. We can then run a simulation of the crystallization process for this powder layer. It can be assumed that each lower layer has a similar temperature profile, but with slightly reduced temperatures due to the powder application for each layer. Thus, one can simulate the crystallization process of a single layer during several laser cycles. Figure 3 presents the simulation of the crystallization degree over 5 cycles where for each new cycle or layer, the temperature was reduced by 2 K.
We see that one layer cannot completely crystallize during the time constraints of one cycle, when this layer is on top of the powder bed. However, crystallization continues throughout the SLS process, as each cycle produces further layers. CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization during several cycles is one of the advantage of SLS, when the resulting 3D object has very strong layer adhesion and isotropic mechanical properties in all directions like hardness, tensile strength and elongation [3].
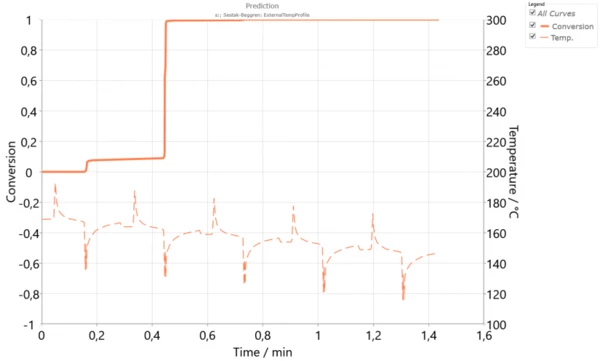
However, if the thickness of the powder layer is increased, then the temperature difference between layers will be higher. This can happen during high-speed SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering. The simulation over 5 cycles with a temperature difference of 5 K (Figure 4) shows that the main crystallization is already finished during the second cycle, while the third layer is already solid. This asynchronous crystallization could be the reason for the mechanical stresses, warpage or curling in the sample because of its shrinkage during the SLS process. Additionally, using thick powder layers could decrease the isotropy of the final material.
Conclusion
The combination of NETZSCH Kinetics Neo with DSC assist in studying the crystallization rate of materials (polymers) and simulate their behavior for such complex industrial processes such as 3D printing by Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering technology. This is very valuable for searching optimal temperature conditions for new materials used in SLS.
Read also / Sources:
- https://ta-NETZSCH.com/how-to-determine-the-process-window-for-sls-powders-using-dsc
- https://ta-NETZSCH.com/how-to-study-the-isothermal-crystallization-behavior-of-sls-powder-using-dsc
- https://3dinsider.com/sls-printing/
- https://doi.org/10.1016/j.tca.2011.03.034
- https://doi.org/10.1016/0040-6031(71)85051-7

FREE E-Book
Thermal Analysis and Rheology in Polymer Additive Manufacturing
Discover the secrets behind AM's game-changing capabilities! Our newly released ebook delves deep into the heart of AM, unveiling the power of reliable material characterization techniques, specifically thermal analysis and rheology.