Glossary
Glass Transition Temperature
The glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.
Many polymers, e.g., thermoplastics, thermosets, rubbers, etc. are usually composed of both amorphous and crystalline structures. This means that many polymers exhibit both a glass transition temperature, Tg, and a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature. The glass transition temperature (Tg) is lower than the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of a crystalline material.

У вас есть вопросы?
Подходящие продукты для вашего измерения
Glass Transition Temperature for Material Identification
Determination of the glass transition temperature is a tool for material identification. The glass transition temperature (Tg) also determines the application field of a material. For example, a rubber tire (car) is soft and ductile because at normal operating temperatures it is well above its glass transition temperature. If its glass transition temperature were higher than its operating temperature, it would not have the flexibility needed to grip the pavement.
Other polymers operate below their glass transition temperature, e.g., a stiff plastic handle. If the plastic handle had a glass transition temperature below its operating temperature, it would be too flexible.
Determination of the glass transition temperature by means of different thermoanalytical methods
by Differential Scanning Calorimetry (DSC)
(e.g., ASTM E1356)
In DSC measurements, the glass transition can be observed by a step in the baseline of the measurement curve (Fig.1). It is characterized by its onset, midpoint, inflection and endset temperature. The step height corresponds to Δcp and is given in J/(g⋅K). The evaluation procedure is described in e.g., ASTM E1356-08. DSC can be used for solids, powders and liquids.
What exactly is Glass Transition Temperature
The glass transition temperature, Tg, of a material characterizes the temperature range over which this glass transition occurs. It is always lower than the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of the crystalline state of the material (if one exists). In the temperature range of the glass transition, polymers change from a hard and rigid state to a more flexible and supple state. Tg occurs in a temperature range over which the mobility of the polymer chains increases significantly.
Thermoplastics like polystyrene (PS) and poly(methyl methacrylate) (PMMA) are usually used below their glass transition temperature, i.e., in their glassy state.
Elastomers like polyisoprene and butadiene rubber (BR) are used above their Tg, where they are soft and supple.
Application
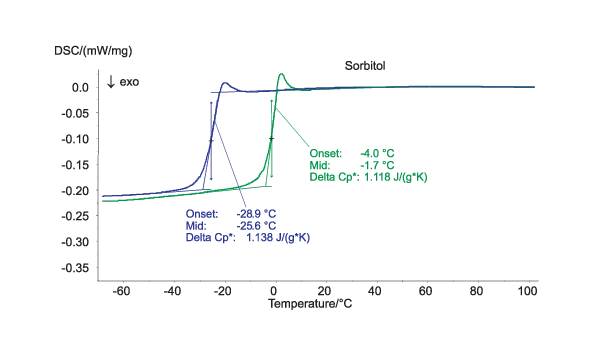
Investigating the Influence of Moisture on the Glass Transition Temperature of Sorbitol
Sorbitol is used as a sugar substitute in many sweets, diet products and medications. A proportion of 10% water in sorbitol effectuates a decrease in the glass transition temperature of approx. 24 K (mid temperatures) relative to anhydrous sorbitol. Both samples remain completely amorphous after the rapid cooling from the molten state (which took place prior to the displayed heating step).
The measurements were carried at a heating rate of 10 K/min in nitrogen atmosphere. The sealed sample pans made of aluminum were closed with a pierced lid. The sample masses amounted to approximately 12 mg ± 1mg.
by Dynamic mechanical analysis (DMA)
(e.g., ASTM 1640)
The DMA technique (e.g., ASTM E1640-09) is a very sensitive technique for the determination of the glass transition temperature (e.g., 1640-94). It provides an alternative procedure in the determination of the glass transition to the use of differential scanning calorimetry (DSC) (ISO 11357‑2). In DMA measurements, Tg can be observed in the extrapolated onset of the sigmoidal change in the storage modulus E’, the peak of the Viscous modulusThe complex modulus (viscous component), loss modulus, or G’’, is the “imaginary” part of the samples the overall complex modulus. This viscous component indicates the liquid like, or out of phase, response of the sample being measurement. loss modulus E’’ and the peak of tanδ.
DMA can be used for unreinforced and filled polymers, foams, rubbers, adhesives and fiber-reinforced plastics/ composites. Different modes (e.g. flexure, compression, tension) of dynamic mechanical analysis can be applied, as appropriate, to the form of the source material.
Application
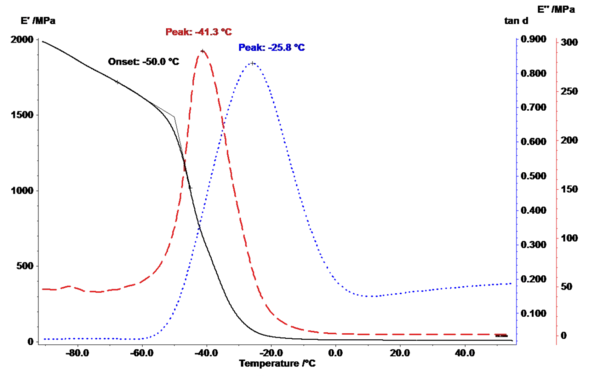
The Glass Transition of a Rubber
Dynamic Mechanical Analysis (DMA) records a material’s temperature-dependent visco-elastic properties (stiffness, E’ and Viscous modulusThe complex modulus (viscous component), loss modulus, or G’’, is the “imaginary” part of the samples the overall complex modulus. This viscous component indicates the liquid like, or out of phase, response of the sample being measurement. loss modulus, E’’, measure for the oscillation energy), and determines its modulus of elasticity and damping values (tanδ) by applying an oscillating force to the sample.
The glass transition temperature, Tg, of a hydrogenated acrylonitrile butadiene rubber (HNBR) was determined in tension mode by means of dynamic mechanical analysis, DMA. The measurement was performed at a heating rate of 2 K/min, a frequency of 1 Hz and an amplitude of ±20µm in the temperature range between -90°C and 40°C. The extrapolated onset determined in the storage modulus E’, the peak in the Viscous modulusThe complex modulus (viscous component), loss modulus, or G’’, is the “imaginary” part of the samples the overall complex modulus. This viscous component indicates the liquid like, or out of phase, response of the sample being measurement. loss modulus E’’ and the peak in the tanδ curve all correspond to the glass transition temperature, Tg, of this rubber material (through application of the respective evaluation conventions).
by Dilatometry (DIL)/ Thermomechanical Analysis (TMA)
(e.g., ASTM E831)
In dilatometer (DIL) and thermomechanical analyzers (TMA, both described in ASTM E 473 – 11a), the glass transition corresponds to the inflection in the dimensional change (e.g., ASTM E1545. It is recorded as the extrapolated onset of kink in the experimental DIL/TMA curve and displayed as a function of temperature. To make this definition reproducible, one should specify the cooling or heating rate. E.g., ASTM E1545 describes the determination of the glass transition by means of TMA.
Application
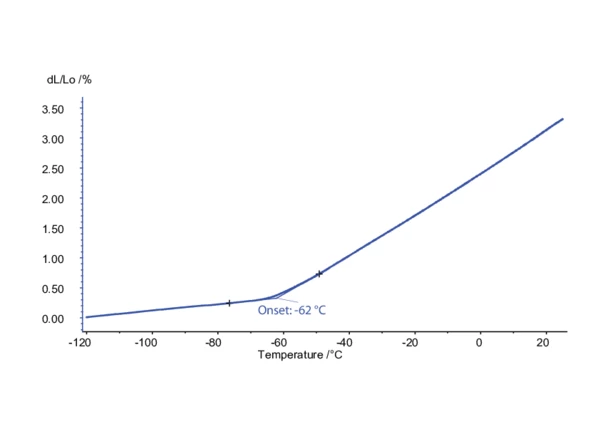
Determination of the Glass Transition by means of Dilatometry
DIL measurement on a natural rubber material between -120°C and 20°C at a heating rate of 3 K/min in helium atmosphere. the sample length amounted to 2 mm. The extrapolated onset temperature of -62°C corresponds to the glass transition (Tg). In amorphous materials such as rubber, it is a reversible transition. The material changes from a hard and relatively brittle state into a soft or rubbery state.



