Glossary
Oxidation
Oxidation can describe different processes in the context of thermal analysis.
To begin with, oxidation is the burning of organic matter in an oxygen-containing atmosphere. Another name for this reaction type is combustion. This can be characterized by TGA and DSC. The TGA curve shows the mass change due to burn-up and the DSC curve shows the corresponding heat of reaction. Oxidation reactions primarily exhibit ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic effects. The heat of combustion cannot be determined by DSC as it is an open system and the gaseous combustion products escape from the crucible. These products can be identified via evolved gas analysis (EGA).
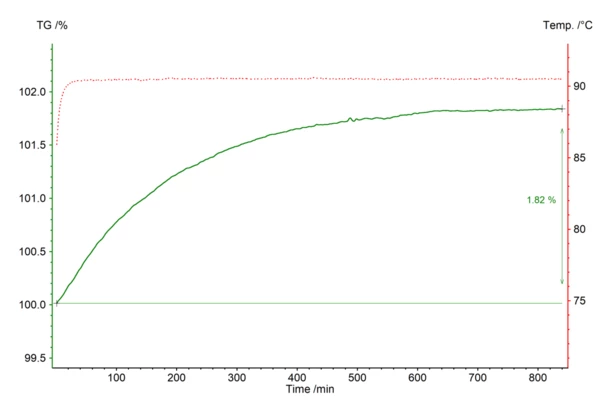
The oxidation of metals can also be investigated by thermal analysis under an oxygen-containing atmosphere. Here, a metal oxide is formed. TGA thereby detects the temperature- or time-dependent mass increase. Oxidation tests on metals in the TGA can also be called corrosion studies. The STA 449 Jupiter® product line enables the measurement of reduction and oxidation cycles via defined changes in the gas atmosphere during the measurement. However, corrosion can be caused not only by oxygen-containing atmospheres, but also by humid atmospheres. Coupling to a humidity generator or a water vapor generator allows the humidity or water vapor at the sample to be defined for a desired temperature. In such cases, the evolved gases can also be identified using EGA.