Introduction
Flame retardants (FRs) have been used for decades to reduce or even eliminate the risk of fire hazards in plastic components for such applications as the electronics or automotive industries. In the early years, halogenated FRs were standard, but more and more, non-halogenated options have been emerging on the market. This is in part due to the added risks of inhaling toxic fumes when halogenated FRs burn, but also due to the changes in regulation and consumer preferences when it comes to sustainability. The most important initiative right now is the EU’s Green Deal, which will result in strong opportunities and, potentially, obligations to transition to halogen- free FRs. This will be even more likely when the anticipated revision of the RoHS (Restriction of Hazardous Substances) takes place.
There are a variety of different solutions and numerous FR polymers available on the market. One of them is expandable graphite, which most only associate with increased thermal and Electrical Conductivity (SBA)Electrical conductivity is a physical property indicating a material's ability to allow the transport of an electric charge.electrical conductivity. However, its unique properties can also be utilized to increase fire safety. To achieve that, large flakes of natural graphite are treated with acids and oxidizing agents. Due to the relatively weak bonds (Van der Waals forces) between the layers as compared to those within a layer, the resulting distance between the layers allows expandable salts to form an intermediate layer – a process called intercalation. These salts expand and drive the individual graphite layers apart when subjected to heat, leading to a huge volume increase. Hereby, expandable graphite combines two modes of fire safety at once. Firstly, the flammability of the component is reduced; and secondly, expandable graphite forms a protective intumescent layer in the case of fire. Therefore, they belong to the class of barrierforming FRs.
Depending on the polymer type , the volume expansion occurs at different temperatures, which limits the group of polymers it can be used for. One of the typical polymers into which FRs are compounded are polyethylenes (PE), which are used for wire and cable sheathing. In this extrusion application, the viscosity of the melt needs to be well controlled to achieve homogeneous thicknesses.
* Intumescent coatings swell when exposed to heat and form an insulating foam that protects the substrate. By EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic reactons, a cooling effect can furthermore be achieved.

Therefore, the amount of flame retardant is critical, because it not only affects the achievable flammability levels, but also processability.
To highlight the effect of different amounts of expandable graphite as a flame retardant on the fire behavior of PE, samples of the different compounds were injection-molded into 100 x 100 x 4-mm3 plates and tested in the TCC 918 (see figure 1). The instrument allows for the determination of the heat release, mass loss and density and composition of the smoke gas.
How is the Measurement Performed
Before starting the tests, the gas analysis system (Siemens Oxymat/Ultramat) was calibrated with calibration gases and the C-factor was checked by using the methane burner with a defined heat release. The gas analyzer used was equipped with O2 and a CO2 option. After heating up the cone heater, the shutter was closed and the horizontal sample holder with the sample was mounted onto the ground plate. Then, the system automatically removed the shutter for the start of the measurement. The evaporated gases were ignited by the automatic ignition system. The measurement conditions are summarized in table 1.
How Are Heat Release, Smoke Density and Mass Loss Connected?
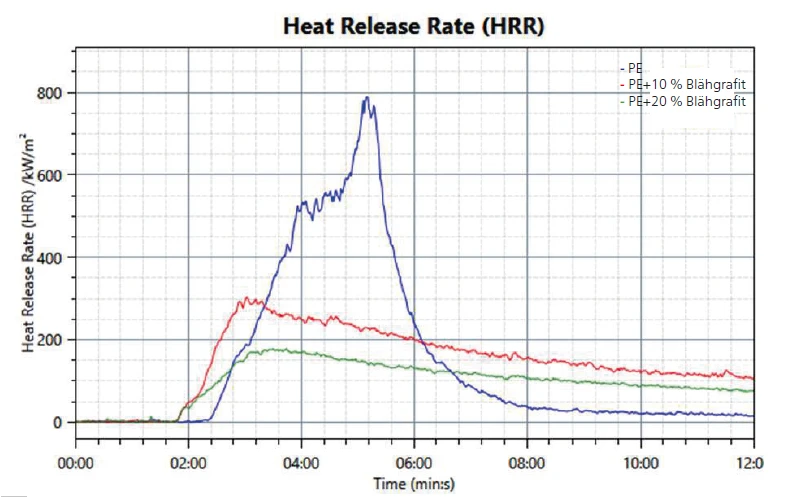
The first effect to be observed is the heat release; see figure 2. While the heat release starts between 2 and 3 minutes after beginning the test for all samples, it can be seen that for PE without any flame retardant (blue line), the heat release increases and reaches a maximum at around 5 minutes. In comparison, both samples with expandable graphite show a much lower heat release and the effect is even stronger with a higher amount of expandable graphite (green line). This points to the barrier properties of the graphite once the intumescent layer has formed.
Table 1: Measurement conditions
| Sample hoder | Horizontal | |
| Heat flux | 50 kW/m² | |
| Nominal duct flow rate | 24.0 l/s | |
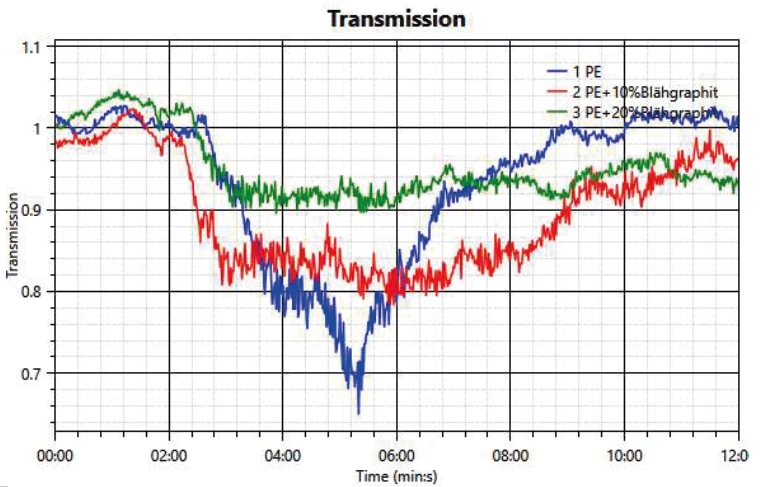
Another important analysis is the development of smoke during a fire. This is measured by detecting a change in transmission, where decreased transmission correlates to increasing smoke density. In figure 3, the measurements of the 3 samples are compared. In all cases, the transmission starts to be reduced after about 2 minutes’ test time. It can be seen that in the case of neat PE, the transmission drops by about 30%. In both samples with FR, the drop is significantly less; the transmission loss is only 20% with 10 wt% expandable graphite and 10% with the higher amount of 20 wt% expandable graphite.
Burning of the sample and the resulting heat release is accompanied by a reduction in weight of the samples. The measured results – see figure 4 – are also in good agreement with the measured heat release and transmission. The highest mass loss is observed for the neat PE sample, followed by the sample with 10 wt% expandable graphite. The lowest mass loss is measured for the sample with the highest amount of FR: 20 wt% expandable graphite.
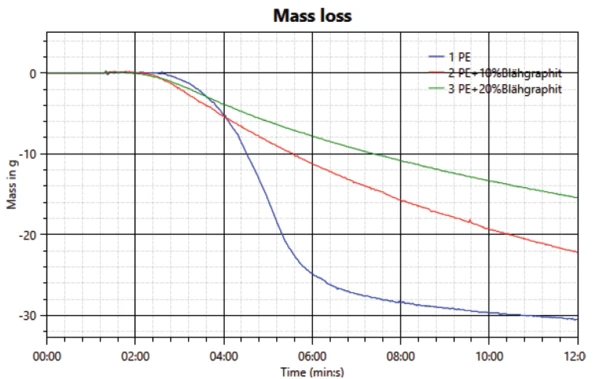
While the beginning of mass loss can be detected after about two minutes, the change in weight first becomes strongly evident when the significant drop in transmission and increase in heat transfer are observed.
What Other Effects Can Flame Retardants Have?
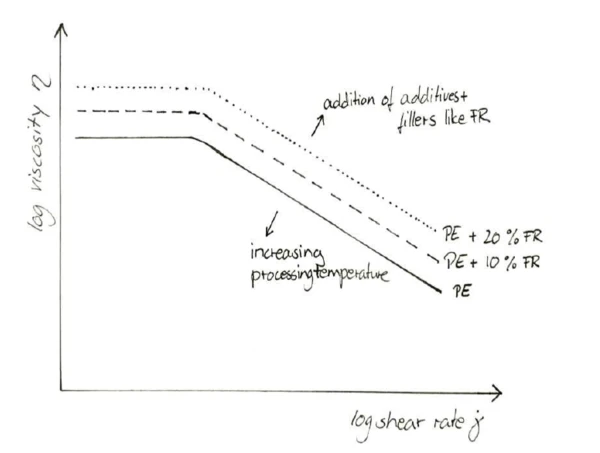
While the higher amounts of FR have a decreasing effect on the heat release, mass loss and increase in transmission property, the change in viscosity needs to be investigated and its effect on the processing behavior evaluated. Just like most additives (excluding flow enhancers), FRs increase the viscosity of the melt across a wide range of shear rates; see figure 5. This can only be balanced to a certain degree by increasing the extrusion temperature. The effect of any given FR amount can be studied in a capillary rheometer as a function of shear rate.
Conclusion
Visually comparing the different samples after the test shows that the untreated PE exhibits significantly more cracks and holes, providing a pathway for oxygen diffusion. It can further be seen that the heat and mass transfer are limited, even as the expandable graphite continues to increase. Thus, it can be concluded that the fire retardancy of expandable graphite results more from a physical than from a chemical action.
The study shows that expandable graphite is a suitable flame retardant for PE and that, within the range of FR content levels investigated here, it is possible to increase the effect by using higher amounts of FR.