Customer SUCCESS STORY
Thermal Analysis at the Max-Planck Institute for Chemical Physics of Solids in Dresden
A Case Study by Susann Scharsach and Dr. Marcus Schmidt about Thermal Analysis Systems Supporting the Synthesis and Crystal Growing at the Max Planck Institute.
The Max-Planck-Gesellschaft is the responsible body for a large number of basic research facilities in Germany and abroad. With its 84 institutes and facilities, it is Germany’s most successful research organization and the international flagship for German science: Along with five institutes abroad, it operates 20 Max Planck Centers with partners like Princeton University in the USA, the Sciences Po University in Paris, France, University College London and the University of Tokyo in Japan.
Max-Planck-Institutes conduct free and independent research in the fields of life sciences, natural sciences and humanities, often on an interdisciplinary basis. With 31 Nobel Prize winners, they are on par with the world’s best and most renowned research institutions.
Source: www.mpg.de

„NETZSCH thermal analysis instruments support the synthesis and crystal growing at the institute. In particular, the Skimmer coupling makes it possible to identify easily condensable gases such as arsenic, tellurium or various metal vapors, even at high temperatures.“
Max-Planck Institute for Chemical Physics of Solids in Dresden
The Max Planck Institute for Chemical Physics of Solids in Dresden (MPI CPfS) was founded in 1995 and includes two chemically and two physically orientated research departments as well as several independent Max Planck research groups with a total of currently 250 employees.

The institute provides findings through experimental research into intermetallic phases and novel chemical, physical and structural properties of substances with metallic and semi-conducting properties. For example, forms of magnetism, superconductivity or metal-semiconductor transitions are investigated. By developing new or alternative synthesis methods, compounds are obtained and subsequently characterized in detail. Insights into how the chemical composition and crystal structure are related to physical properties form the basis for the discovery and understanding of new phenomena in the synthesized compounds. This can be used to develop materials and devices.
MPI CPfS Relies on Solutions by NETZSCH
A central service laboratory for thermal analysis has been operated at the institute for over 20 years. The equipment pool includes two DSC 404 C Pegasus®, two DSC 404 F1 Pegasus®, one STA 409, one DTA 404/7 Cell and one STA 449 F3 Jupiter®. An STA 449 C Jupiter® is installed in an inert gas box from MBraun, while an STA 409 CD, which is coupled to a QMG 422 mass spectrometer via a Skimmer, is also operated in such a box – a solution developed for the institute by NETZSCH in collaboration with MBraun. The devices are equipped with platinum, rhodium, silicon carbide or graphite furnaces. These furnaces cover a temperature range from room temperature to a maximum of 2000°C. Both inert gas atmospheres (argon or helium) and the reactive gases – nitrogen, oxygen or argon/hydrogen – are available for measurement. Easily oxidizable samples or samples in closed metal ampoules are often measured under inert gas. Therefore, when installing and operating the devices, the focus is on a low oxygen partial pressure within the measuring system. This is achieved, among other ways, by using an OTS® system in all devices, by having fixed piping (stainless-steel pipes) in the devices, and by additional cleaning of the inert gases used. Samples that are particularly sensitive to air and/or moisture can be analyzed in the systems integrated in inert gas boxes.

Up to 1500 samples per year are analyzed in our service laboratory. Compounds of virtually all non-radioactive, stable elements except noble gases are analyzed. The particular challenge in most cases is choosing the right crucible or ampoule material. In addition to the many different crucibles offered by NETZSCH, metal ampoules made of tantalum or niobium are often used, which are provided with ceramic inlays made of Al2O3, Y2O3, ZrO2, AlN, BN or glassy carbon, among others. These ampoules were developed and manufactured in the institute's workshop. The ampoules filled with the substances to be measured are welded shut using an electric arc furnace.

Thermal analysis systems support synthesis and crystal growing at the institute by determining melting and solidification temperatures, Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition temperatures, and reaction temperatures; and by analyzing thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition behavior. The Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability and reactivity in different atmospheres are also analyzed. The analytical method is also used in collaboration with other methods to analyze phase diagrams. Thermodynamic data can also be determined. Understanding the thermal behavior is fundamental for achieving the step from compound to applicable material.
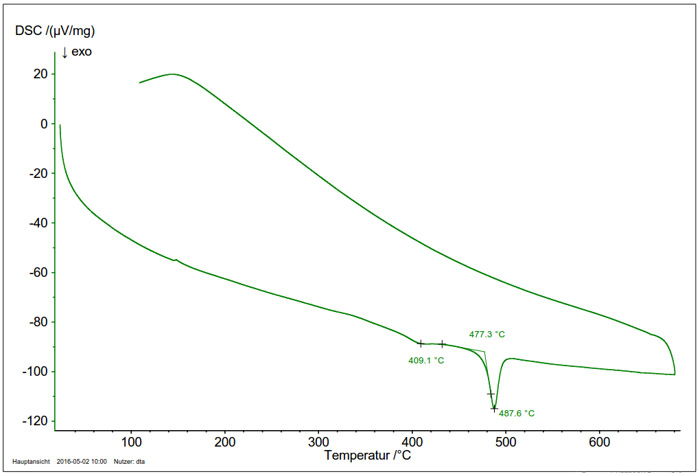
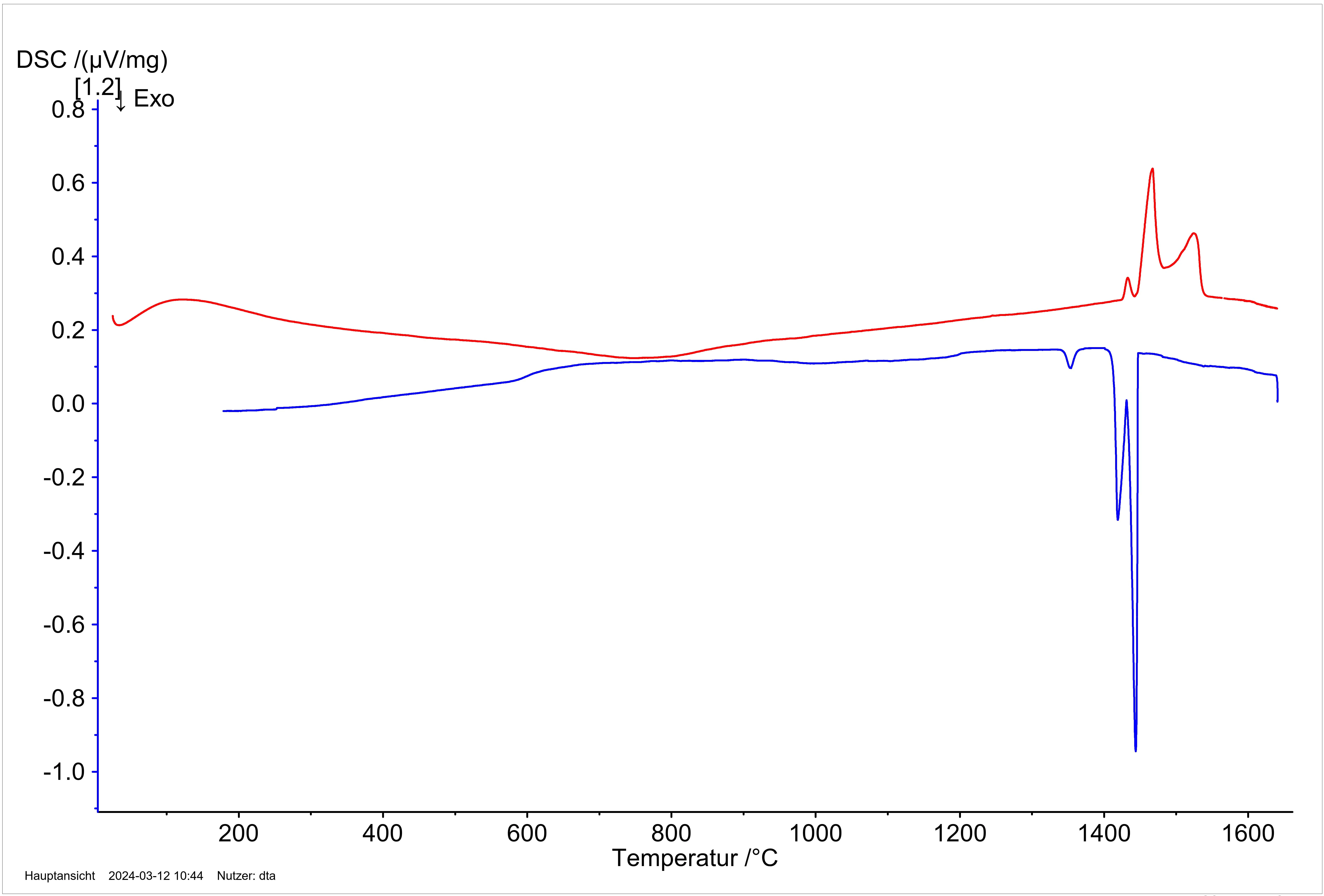
Figure 4: DSC measurement of a Ca/Si high-pressure phase (sample mass 1.5 mg). The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic signal in the heating curve shows the transformation of the metastable high-pressure phase into its thermodynamically stable form.

Unique Instrument Combination
Skimmer Coupling System with Quadrupole Mass Spectrometer and STA
The STA 409 CD with its SKIMMER furnace enabling a direct coupling to the QMG 422 quadrupole mass spectrometer is an important instrument for analyzing the thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition behavior of compounds or the gas phase that are released during chemical reactions. It can be used to identify species that are released simultaneously during Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition and cannot be distinguished using the “indirect” method of thermogravimetry, but can be detected directly in the mass spectrometer.
The system enables measurements up to 1200°C and the detection of gas species up to 512 atomic mass units. In particular, the Skimmer coupling makes it possible to identify easily condensable gases such as arsenic, tellurium or various metal vapors, even at high temperatures.
Another advantage: Because of its high sensitivity, the mass spectrometer can also detect very light substances such as hydrogen or small quantities of vaporizing gas particles thanks to its physical counting method, which contrasts with the weighing method of thermogravimetry.
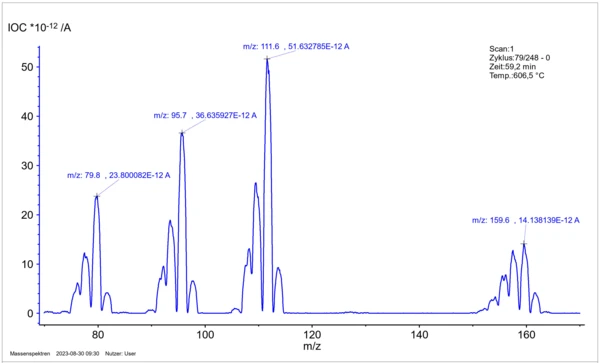
Figure 7: Mass spectrum of the gas phase above Cu2OSeO3 at 606 °C to identify the relevant gas particles based on mass and isotope pattern.
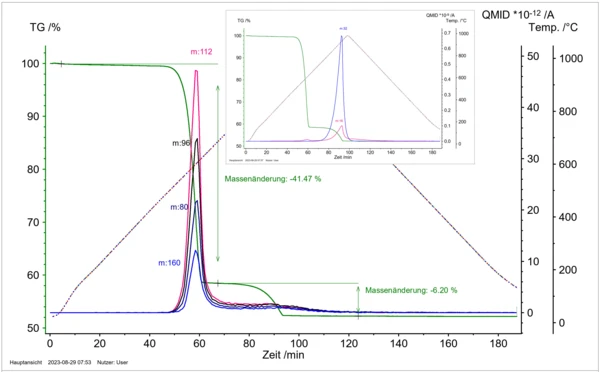
Figure 8: Temperature-dependent mass loss in correlation with the various gas particles detected by mass spectrometry: m/z 16 (O+), 32 (O2+), 80 (Se+), 96 (SeO+), 112 (SeO2+), 160 (Se2+) for the thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of Cu2OSeO3.
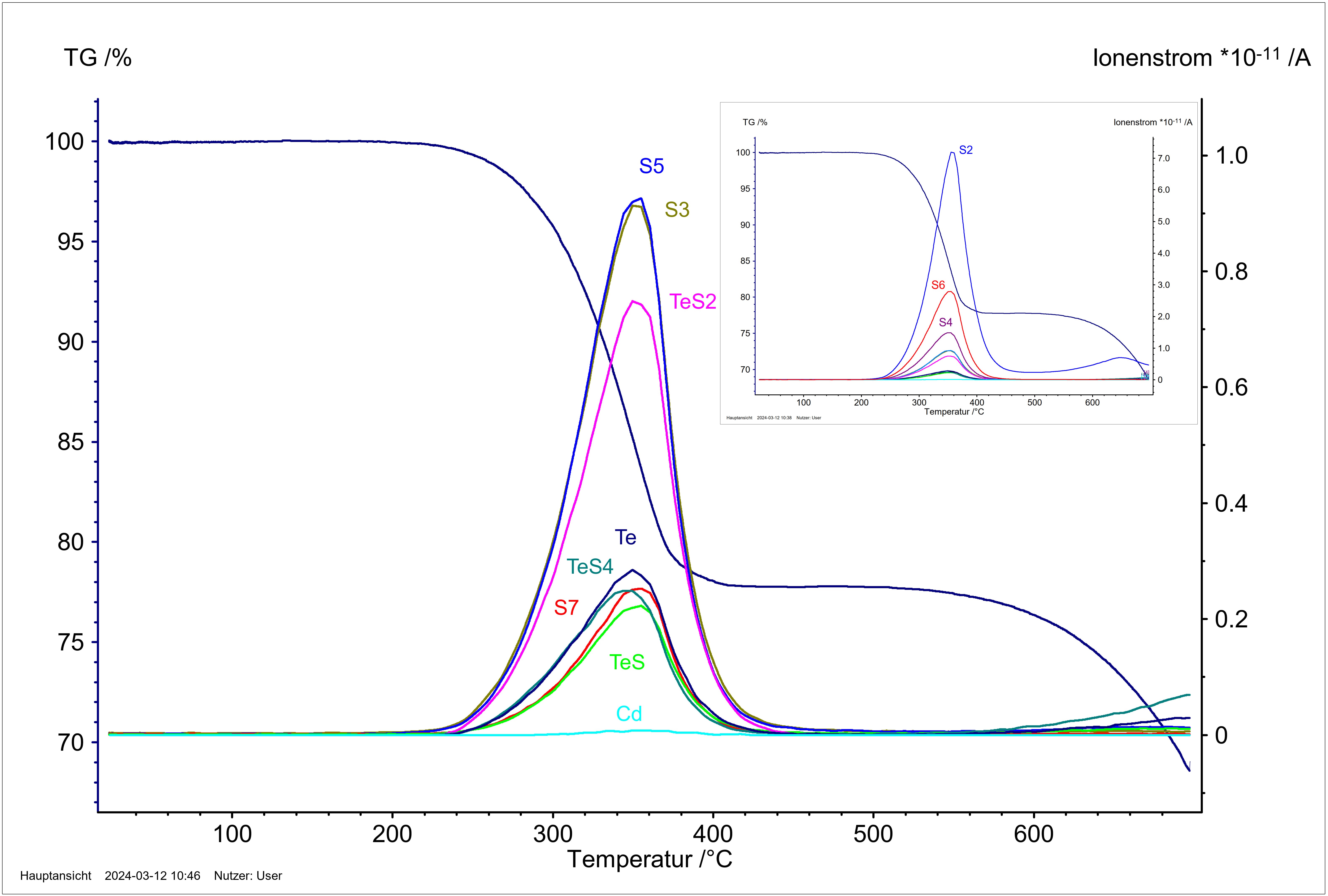
Figure 9: Temperature-dependent mass loss in correlation with the ion current curves of the fragment ions S2+, S6+, S4+, S5+, S3+, TeS2+, Te+, S7+, TeS4+, TeS+ and Cd+. Solid CdTe reacts with sulphur to form solid CdS and release tellurium into the gas phase, whereby no evaporation of cadmium can be observed. The excess sulphur present considerably increases the volatility of tellurium through the formation of Te-S gas species.
We have been working with NETZSCH for 25 years. During this time, we have benefited from excellent customer service and a constant willingness to develop special solutions for our institute.
Susann Scharsach and Dr. Marcus Schmidt
Thank you very much for sharing these interesting insights into your research work. We are looking forward to our continuing our partnership.
About the Authors:
Marcus Schmidt, born in 1967, studied chemistry and completed his doctorate at the Technical University of Dresden on Thermochemical Investigations of Bismuth Oxide Halides. Since 2000, he has been a research associate at the Max-Planck Institute for Chemical Physics of Solids in Dresden where his research topics include Solid-Gas ReactionsSolid-gas reactions are a type of heterogeneous solid-state reaction occurring when a reactive solid is exposed to a stream of reactive gas. Typical examples of solid-gas reactions are sorption and corrosion of metals.solid-gas reactions such as the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of the gas phase and the thermochemical behavior of inorganic materials with a focus on thermal analysis. He is a co-author of the monography “Chemische Transportreaktionen" (with M. Binnewies, R. Glaum, P. Schmidt).
Susann Scharsach, born in 1981, qualified chemical-technical assistant, has worked at the Max-Planck Institute for Chemical Physics of Solids in Dresden since 1999. She played a decisive role in the setup and development of the laboratory for thermal analysis and has significantly contributed to the high quality of the analysis results thanks to her many years of experience.