
20.08.2020 by Dr. Gabriele Kaiser
Efficient Substance Identification Using Identify, …
It is possible to identify substances manually by comparing their characteristic data with literature sources or with previous measurements, but it is far more effective to automatize this process and to use databases for comparison purpose. Learn here how the NETZSCH software can do this for you!
…the NETZSCH Database for Curve Recognition and Comparison of Measurement Curves It is possible to identify substances manually by comparing their characteristic data with literature sources or with previous measurements, but it is far more effective to automatize this process and to use databases for comparison purpose. This procedure has long been common in spectroscopy such as IR and is – since 2013 – also available for thermal analysis evaluations. The corresponding NETZSCH database system is called Identify. Within a few clicks, the current evaluated measurement curve is compared to several libraries (which can be pre-selected). The library material that shows the greatest similarity has the highest probability of being the unknown sample. Libraries can easily be created by users as well as edited and expanded. Possible library entries are measurements and literature data.
Paracetamol – Identification of a Polymorphic Form
In the presented experiment, 1.7 mg of paracetamol, a common analgesic and antipyretic drug substance, was heated to 200°C at 10 K/min in a nitrogen atmosphere using aluminum crucibles. The resulting DSC curve (Fig. 1, in green) shows an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect representing melting of the chemical with a melt onset temperature (extrapolated onset temperature) of 169.0 °C and a melting enthalpy (heat of fusion) of 189.8 J/g. The additionally drawn curve in pink corresponds to the database entry marked in blue (tabular presentation) with the highest similarity. Thus, it is also possible to compare the current measurement curve and the database entry optically. Paracetamol occurs in two polymorphic modifications and both forms differ in their melting range. Therefore, clear assignment via the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point is possible. Here, form I of paracetamol is displayed.
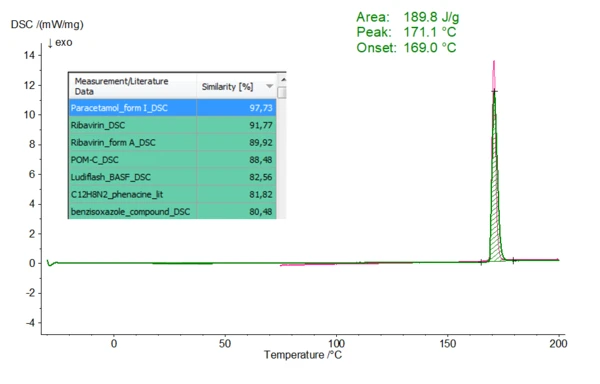
Fig. 1 One-on-one comparison of a DSC measurement on Paracetamol (green) with database entries (measurement conditions of the paracetamol measurement see text). The leading position of the corresponding hit list (marked in blue) depicts the data base entry with the highest similarity. The corresponding DSC curve is shown in pink.