Introdução
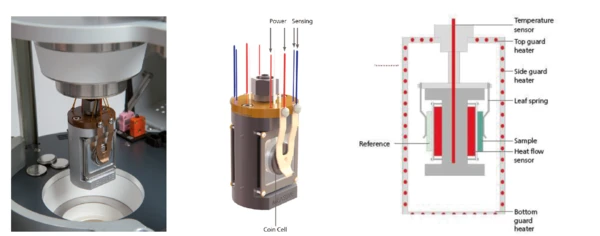
O NETZSCH Multiple Module Calorimeter (Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC®), o ARC®-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC) 274 Nexus® (Figura 1) oferece três módulos de medição diferentes. O Módulo Calorimetria de taxa acelerada (ARC)O método que descreve os procedimentos de teste isotérmico e adiabático usados para detectar reações de decomposição termicamente exotérmicas.ARC® pode ser usado para os chamados testes Heat-Wait-Search (HWS)Heat-Wait-Search é um modo de medição usado em dispositivos de calorímetro de acordo com a calorimetria de taxa acelerada (ARC®).heat-wait-search (Heat-Wait-Search (HWS)Heat-Wait-Search é um modo de medição usado em dispositivos de calorímetro de acordo com a calorimetria de taxa acelerada (ARC®).HWS) ou testes de Fuga térmicaO descontrole térmico é a situação em que um reator químico fica fora de controle em relação à produção de temperatura e/ou pressão causada pela própria reação química. A simulação de um descontrole térmico geralmente é realizada usando um dispositivo de calorímetro de acordo com a calorimetria de taxa acelerada (ARC®).fuga térmica [1][2]; o Módulo de Varredura é adequado para aplicações como a avaliação de Transições de faseO termo transição de fase (ou mudança de fase) é mais comumente usado para descrever transições entre os estados sólido, líquido e gasoso.transições de fase endotérmicas ou exotérmicas, bem como a triagem de risco térmico [3][4]; e o Módulo Coin Cell é especializado na investigação de baterias [5]. Uma unidade externa de ciclo de bateria pode ser facilmente conectada ao Coin Cell Module por meio de um conector LEMO. Os sinais de EstirpeA deformação descreve uma deformação de um material, que é carregado mecanicamente por uma força ou estresse externo. Os compostos de borracha apresentam propriedades de deformação se uma carga estática for aplicada.tensão e corrente podem ser transferidos para o software de avaliação Proteus®; o sinal de potência resultante é automaticamente determinado e quantificado para carga e descarga de forma independente. Ao detectar a perda de calor durante a carga e a descarga, é possível avaliar a eficiência do ciclo de uma bateria. Para isso, o porta-amostras duplo oferece uma configuração diferencial semelhante à DSC (figuras 2a, b, c).

Como a maioria dos estudos não destrutivos de carregamento e descarregamento IsotérmicoOs testes com temperatura controlada e constante são chamados de isotérmicos.isotérmico de baterias é realizada em uma faixa de temperatura muito small próxima à temperatura ambiente, é essencial que o calorímetro seja calibratado de acordo.libraPara a medição de temperatura e sensibilidade, os metais são normalmente usados como materiais de referência.
Temperatura e sensibilidade Calibration
As moedas vazias (figura 3) podem ser usadas de forma semelhante aos cadinhos de DSC para preparar amostras ou materiais de referência. O Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC®), o ARC®-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC Coin Cell Module permite a varredura com taxas de aquecimento moderadas, o que minimiza o deslocamento dinâmico e melhora a comparabilidade com as medições isotérmicas, como as de ciclo de uma bateria.libraOs materiais típicos de medição, juntamente com as massas de amostra correspondentes, estão resumidos na tabela 1.libraA figura 4 mostra um kit de medição estabelecido dessa forma para o Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC®), o ARC®-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC Coin Cell Module.
libraO gálio é um material de seção certificado e bem estabelecido para temperatura e entalpia, recomendado por várias instituições [6]. No entanto, ele raramente é usado porque reage com o alumínio, que é o material do cadinho mais frequentemente usado em DSC. Entretanto, suaTemperaturas e entalpias de fusãoA entalpia de fusão de uma substância, também conhecida como calor latente, é uma medida da entrada de energia, normalmente calor, necessária para converter uma substância do estado sólido para o líquido. O ponto de fusão de uma substância é a temperatura na qual ela muda de estado, passando do sólido (cristalino) para o líquido (fusão isotrópica). temperatura de fusão é apenas ligeiramente superior à temperatura ambiente. Como as moedas são feitas de aço e as taxas de aquecimento aplicadas são comparativamente baixas, as desvantagens mencionadas acima não são relevantes em termos do Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC®), o ARC®-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC Coin Cell Module.

libraTabela 1: Materiais e massas do kit de fabricação do Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC), o ARC-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC Coin Cell Module

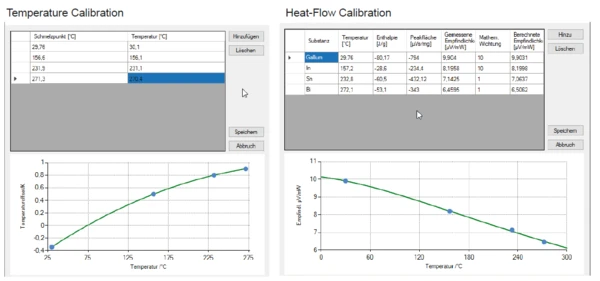
Os resultados do comportamento deTemperaturas e entalpias de fusãoA entalpia de fusão de uma substância, também conhecida como calor latente, é uma medida da entrada de energia, normalmente calor, necessária para converter uma substância do estado sólido para o líquido. O ponto de fusão de uma substância é a temperatura na qual ela muda de estado, passando do sólido (cristalino) para o líquido (fusão isotrópica). fusão dos materiais de referência discutidos acima estão representados na figura 5.libraOs polinômios de ação calculados para a temperatura e a sensibilidade são mostrados na figura 6.libraA fim de verificar novamente os polinômios de variação de temperatura e sensibilidade, foi usado o naftaleno (C10H8).
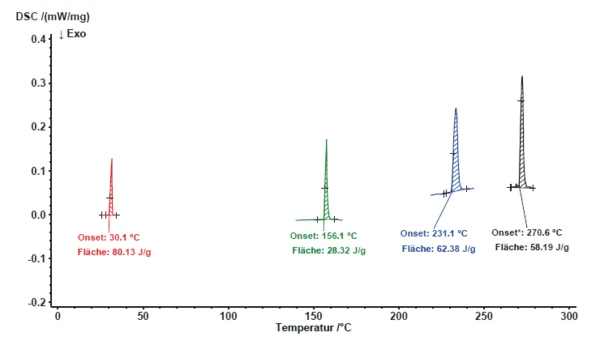
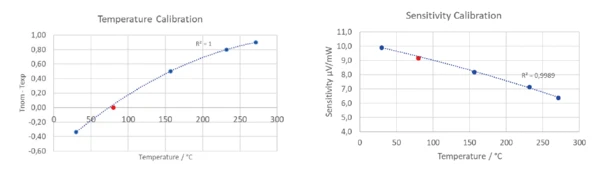
Como os resultados obtidos para o naftaleno estavam de acordo com os polinômios de calibração que foram determinados usando as amostras de metal, eles confirmam a validade da calibração (Figura 7).
Conclusão
Esses resultados demonstram muito bem a capacidade do Calorímetro de múltiplos módulos (MMC)Um dispositivo de calorímetro de modo múltiplo que consiste em uma unidade de base e módulos intercambiáveis. Um módulo é preparado para calorimetria de taxa acelerada (ARC), o ARC-Module. Um segundo módulo é usado para testes de varredura (Scanning Module) e um terceiro está relacionado a testes de bateria para células tipo moeda (Coin Cell Module).MMC Coin Cell Module com relação à temperatura e à entalpia calibration. O uso do gálio como material de calibração é muito importante, uma vez que a calibração adequada próxima à temperatura ambiente é essencial para aplicações de baterias. O ciclo IsotérmicoOs testes com temperatura controlada e constante são chamados de isotérmicos.isotérmico das baterias geralmente é realizado próximo ou ligeiramente acima da temperatura ambiente.libraMateriais de íons de carbono mais comuns, como o índio, por exemplo, com umaTemperaturas e entalpias de fusãoA entalpia de fusão de uma substância, também conhecida como calor latente, é uma medida da entrada de energia, normalmente calor, necessária para converter uma substância do estado sólido para o líquido. O ponto de fusão de uma substância é a temperatura na qual ela muda de estado, passando do sólido (cristalino) para o líquido (fusão isotrópica). temperatura de fusão de 156,6 °C, estariam muito distantes da faixa de aplicação necessária.