Introduction
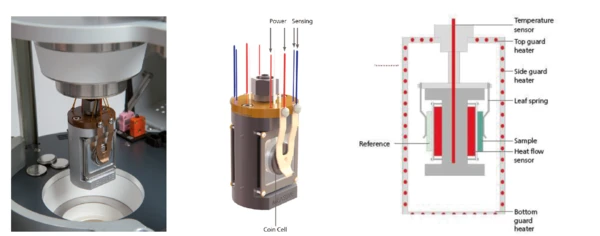
The NETZSCH Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).Multiple Module Calorimeter (MMC) 274 Nexus® (Figure 1) offers three different measurement modules. The Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Module can be used for so-called Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).heat-wait-search (Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).HWS) tests or Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway tests [1][2]; the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module is suited for such applications as the evaluation of EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic or ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transitions as well as thermal hazard screening [3][4]; and the Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module is specialized for the investigation of batteries [5]. An external battery cycling unit can easily be connected to the Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module via a LEMO connector. Signals for voltage and current can be transferred to the Proteus® evaluation software; the resulting power signal is automatically determined and quantified for charging and discharging independently. By detecting the heat loss during charging and discharging, it is possible to evaluate the efficiency of cycling a battery. To this end, the twin sample carrier offers a DSC-like differential setup (figures 2a, b, c).

Since most of the non-destructive IsothermalTests at controlled and constant temperature are called isothermal.isothermal charging and discharging studies of batteries are carried out within a very small temperature range near ambient temperature, it is essential to have the calorimeter calibrated accordingly. For temperature and sensitivity calibration, metals are usually used as reference materials.
Temperature and Sensitivity Calibration
Empty coins (figure 3) can be used in a similar way to DSC crucibles in order to prepare samples or reference materials. The Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module allows for scanning at moderate heating rates, which minimizes the dynamic shift and improves comparability to IsothermalTests at controlled and constant temperature are called isothermal.isothermal measurements such as those for cycling a battery. Typical calibration materials along with the corresponding sample masses are summarized in table 1. A calibration kit established this way for the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module is shown in figure 4.
Gallium is a certified and well-established calibration material for temperature and enthalpy, recommended by several institutions [6]. Nevertheless, it is rarely used since it reacts with aluminum, which is the crucible material most frequently used in DSC. However, its Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature is only slightly above ambient temperature. Since the coins are made of steel and the applied heating rates are comparatively low, the above-mentioned drawbacks are not relevant in terms of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module.

Table 1: Materials and masses of the calibration kit of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module

The results for the melting behavior of the abovediscussed reference materials are depicted in figure 5. The calculated calibration polynomials for temperature and sensitivity are shown in figure 6. In order to doublecheck the calibration polynomials for both temperature and sensitivity, naphthalene (C10H8), was used.
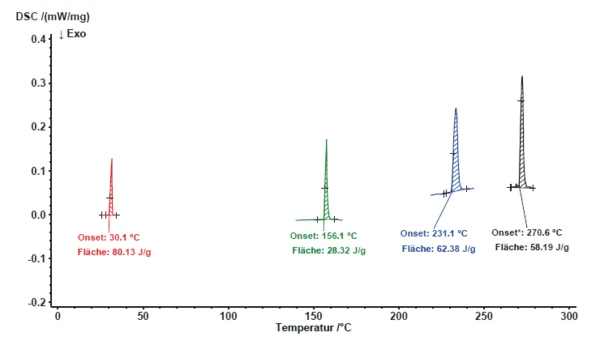
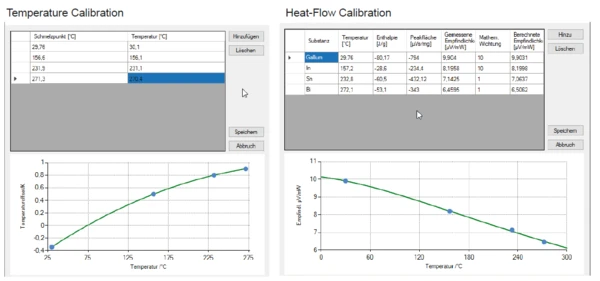
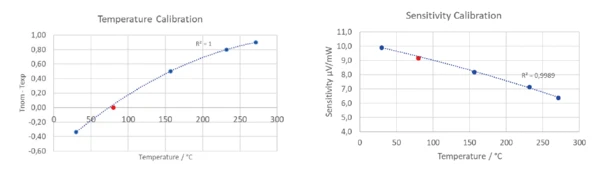
As the results obtained for naphthalene were in good accordance with the calibration polynomials that were determined using the metal samples, these nicely confirm the validity of the calibration (figure 7).
Conclusion
These results nicely demonstrate the capability of the MMC Coin Cell ModuleA calorimeter module being part of the Multiple Module Calorimeter (MMC) allowing for scanning and isothermal tests of complete coins of variable size. The DSC-like twin design gives a differential signal of the heat signature during a heating ramp or charging and discharging of batteries.Coin Cell Module with regard to temperature and enthalpy calibration. The use of gallium as a calibration material is very important since proper calibration near ambient temperature is essential for battery applications. IsothermalTests at controlled and constant temperature are called isothermal.Isothermal cycling of batteries is usually carried out close to or slightly above ambient temperature. More common calibration materials such as indium, for instance, with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of 156.6°C, would be too distant from the required range of application.