
21.01.2020 by Gabriele Stock
Как моделировать и оптимизировать процессы размотки
NETZSCH Менеджер по продукции Елена Мухина объясняет, как программное обеспечение Kinetics Neo помогает быстрее и эффективнее выполнять процессы размотки полимеров.
При обжиге керамики и производстве агломерата качество продукта зависит от температурного профиля, в частности от скорости нагрева. На начальном этапе процесса нагрева полимерное связующее тщательно удаляется путем термического разложения. Но развитие газа не должно быть слишком интенсивным, чтобы предотвратить образование микротрещин и гарантировать, что структура исходного материала не будет разрушена. Поэтому, чтобы получить продукт наилучшего качества, этот этап нагрева для разложения полимера не должен выполняться слишком быстро. С другой стороны, чрезмерно медленный нагрев увеличивает время процесса, что может быть чрезмерно дорогостоящим и экологически небезопасным, а также увеличивает производственные затраты. Основная задача - создать оптимальный температурный профиль со сбалансированным нагревом, чтобы обеспечить наилучшее качество материала за минимальное время. Для достижения этой цели необходимо знать, что происходит с материалом в печи во время обжига. Рекомендуется использовать метод моделирования для определения скорости связывания для заданного температурного профиля печи. Данные для анализа Полимерные связующие теряют массу при термическом разложении, что можно легко измерить с помощью термогравиметрии. Однако химические реакции - это кинетические процессы, зависящие не только от температуры, но и от времени. Так, при постоянной температуре реакция будет протекать, и масса будет меняться, однако одна и та же масса может возникать при разных температурах. Единственное, что не зависит от температурного профиля, - это химические свойства реакции, такие как стехиометрические коэффициенты, порядки реакций и энергии активации. При наличии связующего компоненты полимерной смеси часто разлагаются независимо друг от друга. В этом случае начальный и конечный составы материала обычно не зависят и от температурного профиля. Для того чтобы найти те параметры химических реакций, которые не зависят от температурного профиля, необходимо провести несколько термогравиметрических лабораторных измерений при разных температурных условиях, а именно при разных скоростях нагрева. Типичная форма термогравиметрических кривых разложения показывает температурную зависимость от измеряемой массы для различных скоростей нагрева. Представленные здесь измерения проводились на приборе NETZSCH TG 209 F1 . Кинетический анализ В полимерном связующем полимеры обычно разлагаются независимо друг от друга, и измеренная потеря массы отражает процесс разложения смеси. Каждый компонент в смеси может распадаться в несколько отдельных этапов, таким образом, отдельные этапы этого процесса могут относиться к разным полимерам или к одному и тому же полимеру. Кинетический анализ позволяет найти кинетические параметры наблюдаемого процесса, которые не зависят от температурного профиля. Этими параметрами являются энергия активации и порядок реакции для каждой видимой стадии разложения, а также вклад каждой стадии реакции в общий процесс разложения. Существует два различных подхода к кинетическому анализу измеренных данных - первый основан на модели в соответствии с реальной химией процесса и рассматривает общий процесс как сумму независимых процессов разложения различных полимеров. Разложение каждого полимера рассматривается как серия последовательных индивидуальных реакционных шагов. При этом каждый этап реакции имеет свою стехиометрию и энергию активации, которые сохраняют постоянные значения от начала этапа реакции до его завершения. Такой подход описывает процесс разделения явно и очень близко к реальности, но требует времени на анализ и построение кинетической модели из параллельных и последовательных шагов реакции. Второй, более приближенный подход называется безмодельным, когда весь процесс рассматривается как одностадийная реакция, в которой энергия активации и предэкспоненциальные коэффициенты изменяются по мере протекания реакции. Этот тип очень быстр для процессов с последовательными стадиями, но также имеет некоторые ограничения, например, он не может описать разложение смеси с параллельными реакциями или с реакциями, имеющими значительное перекрытие. NETZSCH Программное обеспечение Kinetics Neo поддерживает оба метода анализа, что является преимуществом перед программами, использующими один метод. NETZSCH Kinetics Neo использовалась для анализа термогравиметрических данных методом, основанным на модели, в результате чего была получена кинетическая модель, изображающая три последовательных этапа реакции с их кинетическими параметрами. Эта модель не зависит от температурной программы и может быть использована для моделирования процессов разложения при других заданных пользователем температурных программах. Если моделирование проводится точно для тех же температур, которые использовались во время эксперимента, то смоделированные кривые должны соответствовать эксперименту, если модель верна. Такое соответствие видно на рисунке, где экспериментальные данные для разных скоростей нагрева отмечены символами, а все смоделированные данные, основанные на одной и той же кинетической модели с тем же набором кинетических параметров, но для разных скоростей нагрева, показаны сплошными кривыми. Это означает, что кинетическая модель была построена правильно, и кинетические параметры оказались верными, поэтому данная модель может быть использована в будущем для моделирования разложения связующего внутри печи, где невозможно измерить потерю массы. Прогнозирование и оптимизация Полученная кинетическая модель, состоящая из трех отдельных последовательных этапов реакции, позволяет прогнозировать потерю массы для заданной пользователем температурной программы. Таким образом, зная, насколько горячо внутри печи, можно моделировать ход процесса дебридинга. Например, эта модель позволяет моделировать потерю массы материала в туннельной печи. В случае изменения температуры программа рассчитывает новую кривую потери массы для новой температурной программы в каждой зоне. Скорость разложения зависит не только от температуры, но и от текущего значения конверсии. При постоянной скорости нагрева на кривой потери массы есть диапазоны, где процесс идет быстро, и диапазоны, где процесс идет медленно. Параметры с высокой скоростью реакции являются зонами риска, где структура материала может быть повреждена. Диапазоны с низкой скоростью реакции приводят к неоправданным потерям времени и энергии, а значит, к слишком высокой стоимости конечного продукта. Для оптимизации процесса необходимо найти такие температурные профили, при которых скорость потери массы будет постоянной, чтобы получить оптимальное качество продукта за минимальное время. Без возможности моделирования такие температурные профили инженеру-химику пришлось бы создавать методом проб и ошибок - это потребовало бы значительного времени и привело бы к существенным затратам. С помощью программы Kinetics Neo был рассчитан новый температурный профиль для заданной скорости потери массы 0,05 %/мин. В промышленных процессах, характеризующихся некоторыми ограничениями в скорости нагрева, это программное обеспечение может помочь найти оптимальный температурный профиль для получения моделируемой скорости потери массы, которая очень близка к постоянному значению. Например, немецкой компании Haldenwanger потребовалось программное обеспечение для оптимизации температурного профиля при обжиге керамики в отношении ее новой пенокерамики, качество которой очень чувствительно к скорости обжига. Этот процесс состоит из двух частей: обжиг и спекание. Оптимизация температурного профиля была проведена для обеих частей, и время производства сократилось более чем на 50 %. Применение программного обеспечения для кинетического анализа В полимерном связующем полимеры обычно разлагаются независимо друг от друга, и измеренная потеря массы отражает процесс разложения смеси. Каждый компонент в смеси может распадаться в несколько отдельных этапов, таким образом, отдельные этапы этого процесса могут относиться к разным полимерам или к одному и тому же полимеру. Кинетический анализ позволяет найти кинетические параметры наблюдаемого процесса, которые не зависят от температурного профиля. Этими параметрами являются энергия активации и порядок реакции для каждой видимой стадии разложения, а также вклад каждой стадии реакции в общий процесс разложения. Существует два различных подхода к кинетическому анализу измеренных данных - первый основан на модели в соответствии с реальной химией процесса и рассматривает общий процесс как сумму независимых процессов разложения различных полимеров. Разложение каждого полимера рассматривается как серия последовательных индивидуальных реакционных шагов. При этом каждый этап реакции имеет свою стехиометрию и энергию активации, которые сохраняют постоянные значения от начала этапа реакции до его завершения. Такой подход описывает процесс разделения явно и очень близко к реальности, но требует времени на анализ и построение кинетической модели из параллельных и последовательных шагов реакции. Второй, более приближенный подход называется безмодельным, когда весь процесс рассматривается как одностадийная реакция, в которой энергия активации и предэкспоненциальные коэффициенты изменяются по мере протекания реакции. Этот тип очень быстр для процессов с последовательными стадиями, но также имеет некоторые ограничения, например, он не может описать разложение смеси с параллельными реакциями или с реакциями, имеющими значительное перекрытие. NETZSCH Программное обеспечение Kinetics Neo поддерживает оба метода анализа, что является преимуществом перед программами, использующими один метод. NETZSCH Kinetics Neo использовалась для анализа термогравиметрических данных методом, основанным на модели, в результате чего была получена кинетическая модель, изображающая три последовательных этапа реакции с их кинетическими параметрами. Эта модель не зависит от температурной программы и может быть использована для моделирования процессов разложения при других заданных пользователем температурных программах. Если моделирование проводится точно для тех же температур, которые использовались во время эксперимента, то смоделированные кривые должны соответствовать эксперименту, если модель верна. Такое соответствие видно на рисунке, где экспериментальные данные для разных скоростей нагрева отмечены символами, а все смоделированные данные, основанные на одной и той же кинетической модели с тем же набором кинетических параметров, но для разных скоростей нагрева, показаны сплошными кривыми. Это означает, что кинетическая модель была построена правильно, и кинетические параметры оказались верными, поэтому данная модель может быть использована для будущего моделирования разложения связующего внутри печи, где нет возможности измерить потерю массы.
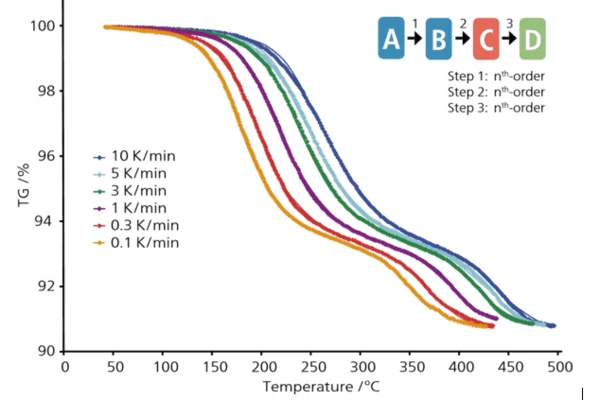
Прогнозирование и оптимизация Полученная кинетическая модель, состоящая из трех отдельных последовательных этапов реакции, позволяет прогнозировать потерю массы для заданной пользователем температурной программы. Таким образом, зная, насколько горячо внутри печи, можно моделировать ход процесса обдирки. Например, эта модель позволяет моделировать потерю массы материала в туннельной печи. В случае изменения температуры программа рассчитывает новую кривую потери массы для новой температурной программы в каждой зоне.
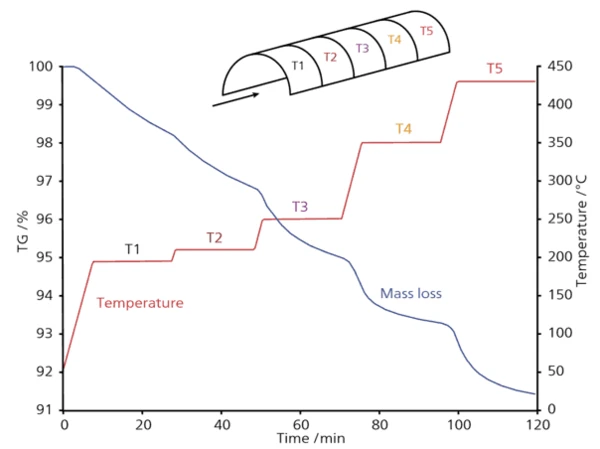
Скорость разложения зависит не только от температуры, но и от текущего значения конверсии. При постоянной скорости нагрева на кривой потери массы существуют диапазоны, где этот процесс протекает быстро, и диапазоны, где он протекает медленно. Параметры с высокой скоростью реакции являются зонами риска, где структура материала может быть повреждена. Диапазоны с низкой скоростью реакции приводят к неоправданным потерям времени и энергии, а значит, и к слишком высокой стоимости конечного продукта. Для оптимизации процесса необходимо найти такие температурные профили, при которых скорость потери массы будет постоянной, чтобы найти оптимальное качество продукта за минимальное время. Без возможности моделирования такие температурные профили инженеру-химику пришлось бы создавать методом проб и ошибок - это потребовало бы значительного времени и привело бы к существенным затратам. С помощью программы Kinetics Neo был рассчитан новый температурный профиль для заданной скорости потери массы 0,05 %/мин.
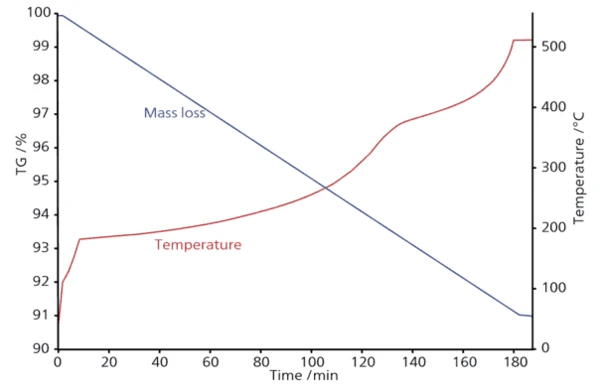
В промышленных процессах, характеризующихся некоторыми ограничениями в скорости нагрева, это программное обеспечение может помочь найти оптимальный температурный профиль для получения моделируемой скорости потери массы, которая очень близка к постоянному значению. Например, немецкой компании Haldenwanger потребовалось программное обеспечение для оптимизации температурного профиля при обжиге керамики в отношении ее новой пенокерамики, качество которой очень чувствительно к скорости обжига. Этот процесс состоит из двух частей: обжиг и спекание. Оптимизация температурного профиля была проведена для обеих частей, и время производства сократилось более чем на 50 %. Приложения программного обеспечения для кинетического анализа Область применения кинетического анализа и моделирования не ограничивается процессом размола при производстве керамики или в агломерационной металлургии. Такое моделирование необходимо, например, для определения срока службы упаковочных материалов или для технологических операций при высоких температурах.