금속 / 합금
Melting Point Determination on Palladium
The largest use of palladium (Pd) today is in catalytic converters.
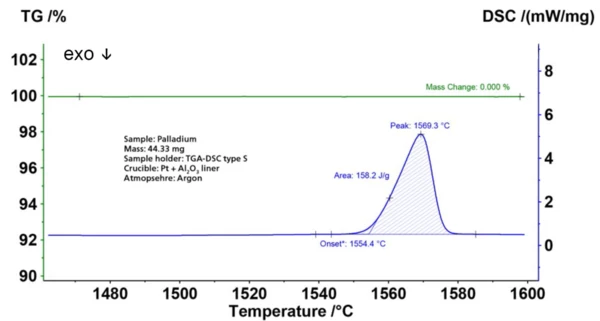
However, it is also used in e.g., dentistry, watch making, blood sugar test strips, aircraft spark plugs and in the production of surgical instruments and electrical contacts. Palladium shows no reaction with oxygen at normal temperature although when heated to 800°C in air will produce a layer of palladium(II) oxide (PdO). This plot exhibits the STA measurement on Pd up to a sample temperature of 1600°C under argon atmosphere. The DSC curve (blue) shows the melting with an enthalpy of 158 J/g (blue curve, DSC) at 1554°C (onset temperature). Both values correspond very well with literature data (< 1%) for pure Pd. Before and after melting no mass loss occurred (green curve); this confirms the high purity of the metal as well as the vacuum-tightness of the STA 449 F5 Jupiter®®.