
28.11.2021 by Dr. Carolin Fischer, Dr. Natalie Rudolph, Milena Riedl
Parchment Paper and Silicone Baking Mat – Are They Safe to Use?
The Easter season of the year is for many the season for baking cookies and biscuits. But then, we have to decide: parchment paper or silicone baking mat? Today, we are answering the question if the two options are safe to use.
This season of the year is for many the season for baking cookies and biscuits. Butter. Sugar. Flour. Mixer. Rolling Pin. Cookie Cutter… But then, we have to decide: Parchment paper or silicone baking mat?
Many prefer the silicone baking mat due to its non-stick baking surface made of food-grade silicone and the fact that it is reusable ‒ unlike parchment paper which is a single-use item.
Silicones or silicone rubbers belong to the class of cross-linked polymers. The backbone of any silicone is based on alternating silicon (Si) and oxygen (O) atoms; two organic groups are bound to each Si atom. The most common silicone is polydimethylsiloxane (PDMS). Depending on their molecular structure and crosslinking DensityThe mass density is defined as the ratio between mass and volume. density, such silicones range in physical form from rigid to flexible. They have many great properties that make them suitable for a variety of technical applications; however, their excellent heat resistance of up to 260°C ‒ and some grades even above 300°C ‒ make them a great choice for baking molds, mats and spatulas.
However, most customers and our analysis experts in the NETZSCH Applications Laboratory wonder if they are safe to use?
During vulcanization, cyclic and linear siloxane oligomers are common by-products. Therefore, it is useful to study whether any of the processing aids or by-products are released during the baking process, as well as to detect potentially hazardous outgassing during heating – e.g., the release of plasticizers or toxic PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis products – which may be transferred to the baked goods.
Thermal Analysis is suitable for determining product safety
Thermal analysis can be used to detect the release of substances during the baking process. By means of TGA-FT-IR analysis, it is possible to identify the type and temperature of release.
To answer this question, our TGA-FTIR specialists cut a silicone baking mat and silicone-coated parchment paper into pieces and put several pieces of each material into the crucible. The measurements were performed with a PERSEUS® TGA 209 F1 Libra® at a temperature of 230°C, maximum temperature for this mat.
| Sample mass | ~ 130 mg (both samples) |
| Temperature program | RT – 230 °C, held constant for 60 min |
| Heating rate | 10 K/min |
| Atmosphere | Air |
Table 1: Measurement conditions
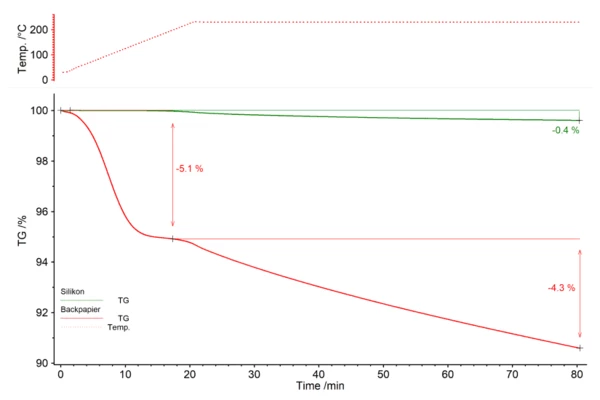
Determining the mass loss in the two samples
The silicone baking mat (green) loses 0.4% of its mass during the heating cycle. The parchment paper (red), on the other hand, loses 5.1% of its initial mass during heating and a further 4.3% during IsothermalTests at controlled and constant temperature are called isothermal.isothermal treatment. The mass loss is not completed after 60 minutes in either of the two cases.
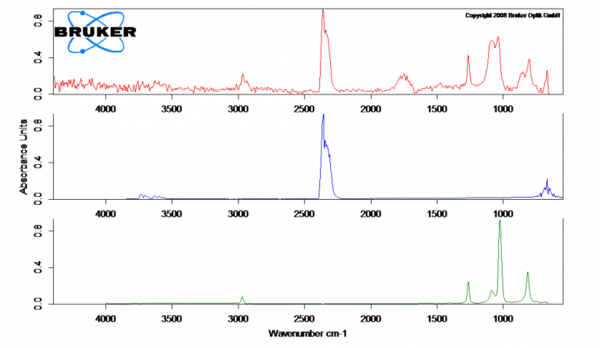
Analyzing the gases evolved from the silicone baking mat
The evolving gases are transported directly from the thermobalance to the FT-IR above by means of the coupling and identified in the spectrometer gas cell.
The escaping gases are transferred identified by means of Fourier Transform Infrared Spectrometry (FT-IR). In the case of the silicone mat (spectrum at 230°C in red), the release of CO2 (blue) and traces of the degradation products of silicone rubber (green) were found, which could also be oligomer by-products from production. More details about the individual components released could be provided by GC-MS coupling.
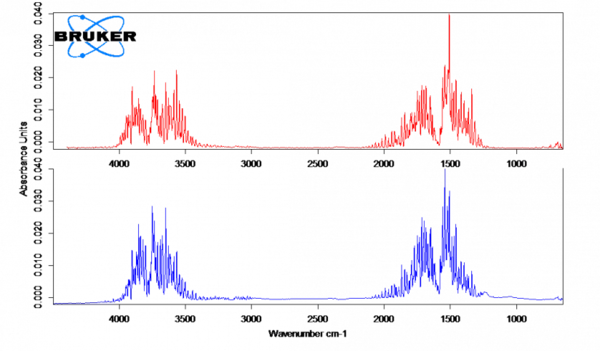
Analyzing the gases evolved from the parchment paper
Parchment paper only releases water, which occurs in the temperature range up to 150°C. Figure 3 shows the measured spectrum at 100°C in red in comparison with the database spectrum for water (blue).
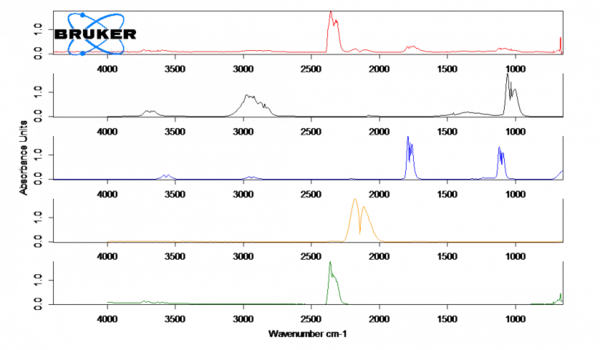
The spectrum for parchment paper (red) at 230°C shows the release of CO (orange), CO2 (green) and small traces of methanol (black) and formic acid (blue). These Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products are presumably produced by the thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the paper. After the measurement, a brown discoloration of the paper can be observed.
Conclusion
Outgassing of water, CO and CO2 is harmless as they leave the oven in gaseous form. The amounts of methanol and formic acid contained are likely very small. In addition, the silicone degradation products are probably also insignificant since they are harmless. Nevertheless, heating a silicone baking mat without baking goods prior to its first use would certainly be a sensible action.
We here at NETZSCH Analyzing & Testing hope these insights will help you enjoy your freshly baked cookies even more this Easter season. We certainly do now!