
10.08.2023 by Dr. Gabriele Kaiser
How Does Cotton Get into a Lipstick?
Whether in Chanel, Dior, Estée Lauder, Babor, Lancôme or Douglas, hydrogenated cottonseed oil is a substance that is increasingly found on the list of ingredients for decorative cosmetics and personal care products. Go on reading to find out what’s behind this name and how the heating and cooling behavior of this additive can be determined by means of the new DSC 300 Caliris® Classic by NETZSCH.
Cottonseed oil seed is extracted from the seeds of the cotton plant [1] and is valued as an edible oil in many countries. Since the cotton plant contains a natural toxin against insect feeding, the oil must first be refined and the harmful gossypol must be removed. This results in a pale yellow liquid with a high content of unsaturated fatty acids and vitamin E.
Due to its high stability, cottonseed oil is frequently used in cosmetic products in its hydrogenated form. The term hydrogenation describes the accumulation of hydrogen to unsaturated double bonds in the presence of a catalyst and is also referred to as “hardening”. Through hydrogenation, the light yellow oil turns into a white or almost white powder. This process, however, usually leaves some unsaturated bonds. Therefore, in addition to 94 % saturated fat, hydrogenated cottonseed oil usually still contains about 2% unsaturated fatty acids [2].
As a cosmetic ingredient, hydrogenated cottonseed oil features moisturizing properties and a non-greasy texture; it leaves the skin feeling smooth and soft [3]. It can be found in skin cleansing products, lip liners, eyeliners and lipsticks, among others.

Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization Behavior
The NETZSCH DSC 300 Caliris® Classic was employed for the investigations detailed here. By virtue of its small footprint, it fits into (almost) every laboratory.
Like all oils and fats, hydrogenated cottonseed oil belongs to the lipid group and consists of triglycerides of various fatty acids, among them palmitic acid and stearic acid. The melting range of lipids depends on many different factors such as chain length, chain branching, number of double bonds, degree of esterification, and arrangement in the crystal structure [4], since fats and oils can exist in different polymorphic forms or modifications.
In the current case, the sample shows a broad melting range between approx. 35°C and 74°C upon heating (fig. 1, 1st heating, blue curve).
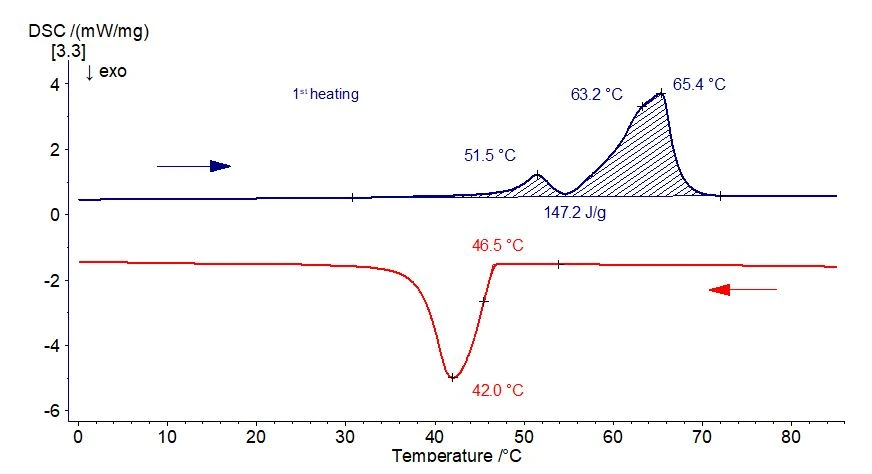
Several EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effects can be seen in this temperature range: the most significant are at about 52°C, 63°C and 65°C (peak temperature in each case).
During the subsequent controlled cooling (red curve in figure 1), the substance starts to crystallize at approx. 47°C. The solidification effect is not structured.
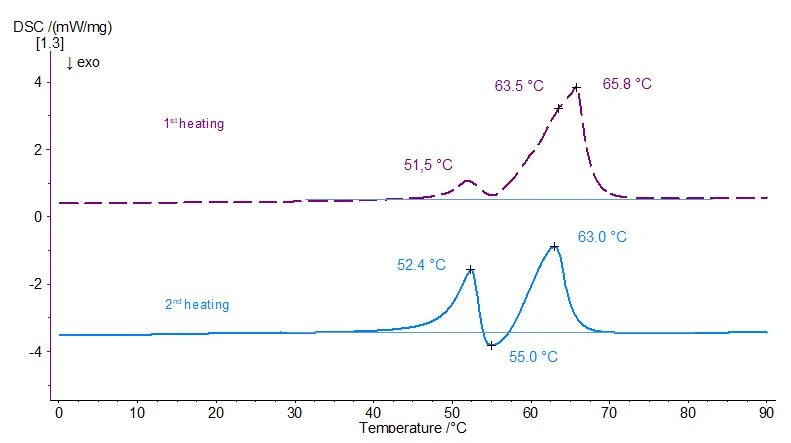
If the sample is heated a second time after cooling (again at a heating rate of 10 K/min, light blue curve in figure 2), a completely different picture is obtained than in the 1st heating, reflecting the polymorphic character of the hydrogenated cottonseed oil. Along with two distinct endothermal effects at 52°C and 63°C (peak temperature in each case), an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic effect occurs in between at approx. 55°C (also peak temperature). The temperature position of the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect at 52°C (light blue curve in figure 1) agrees well with the corresponding EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect in the 1st heating (dashed violet curve). The second EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic peak seems to have shifted slightly to the left as compared to the 1st heating.
By varying the heating rate during the 2nd heating, it is possible to completely suppress the first EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect and to separate the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic peak from the second EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect at low heating rates (2 K/min, light blue curve in figure 3). At higher heating rates (5, 10 or 20 K/min), the first endothermal effect occurs and becomes more and more dominant with increasing heating rate until the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic is entirely overcompensated at a heating rate of 20 K/min.
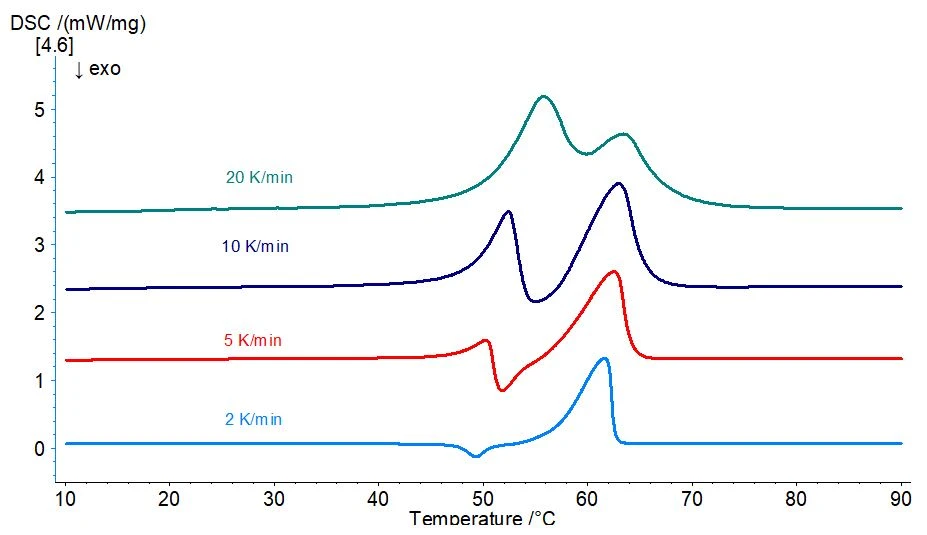
It is therefore possible that the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic peak in the range from 50°C to 55°C is based on a structural change. Further investigations using such techniques as X-ray structural analysis would be necessary to verify this hypothesis.
Conclusion
Hydrogenated cottonseed oil is a hydrogenated vegetable oil, the rather complex melting behavior of which can be described phenomenologically in a fast and straightforward manner by means of the DSC 300 Caliris® Classic. It can be used in cosmetics and creams as an alternative to hard waxes [5].
Literature:
[1] https://www.cosmeticsinfo.org/ingredients/hydrogenated-cottonseed-oil/
[2] https://en.wikipedia.org/wiki/Cottonseed_oil
[4] C. Reitz, PhD thesis, Extrudierte Fettmatrizes mit retardierter Wirkstofffreigabe, Heinrich-Heine-Universität Düsseldorf, 2007, pp 11 – 13
[5] https://file.wuxuwang.com/hpe/HPE6/HPE6_326.pdf
The future is now! Bring our devices into your laboratory with the click of a button.
Simply scan the QR code and get a 3D model of the instrument directly on your mobile phone or tablet. With the help of the latest AR Technology (Artificial Reality), the 3D model can easily be placed in your laboratory in its original life size. This function is browser-based and requires no app.

