Introduction
Glass as a material is omnipresent in our everyday life. Be it as window panes, reading glasses, wine glasses or in the electronic components of our mobile phones – the application areas for glass are versatile and manifold. Basically, glasses are amorphous solids that do not have an atomic long-range structural order. The most widely used glasses primarily consist of inorganic oxide compounds such as silicon dioxide (SiO2) and sodium oxide (Na2O) as well as other admixtures [1]. The mixing ratios – or the purity of the components – determine the properties and thus the application range.
Pure silicate glass, also called fused silica, is a special type of glass consisting of highly pure silicon oxide and not containing any significant impurities. Compared to other inorganic glasses, it features high-temperature resistance, low thermal expansion, chemical resistance and biocompatibility as well as high optical transparency – ranging from ultraviolet to infrared [2]. This material finds applications in various fields, including serving as viewing glasses in high-temperature environments, functioning as lenses in laser systems, supporting implantology procedures, and being utilized in analytical instruments like dilatometers.
Measurement of the Thermomechanical Properties of Glasses by Means of DMA
Dynamic-mechanical analysis (DMA for short) is an experimental method for investigating the viscoelastic properties of materials. This involves analyzing the material’s response to periodic mechanical loads in order to determine properties like elasticity, viscosity and damping. The DMA 303 Eplexor® is a dynamic-mechanical desktop instrument allowing for total force levels of up to 50 N. The system features a temperature range of -170°C to 800°C, which is unique for bench-top instruments. Based on these properties, both materials in the low-temperature range, such as polymers, and highly stiff materials, such as steels, ceramics or glasses, can be characterized to temperatures of 800°C.
Measurement Results
Figure 1 compares the DMA measurement on a conventional float glass (blue curve), as employed in house windows, with that of pure fused silica (red curve) from 100°C to 800°C. The measurement was carried out in 3-point bending with a free bending length of 20 mm and a frequency of 1 Hz. The cubic samples have a thickness of 1 mm and a width of 10 mm with the samples’ outer contour having been smoothed.
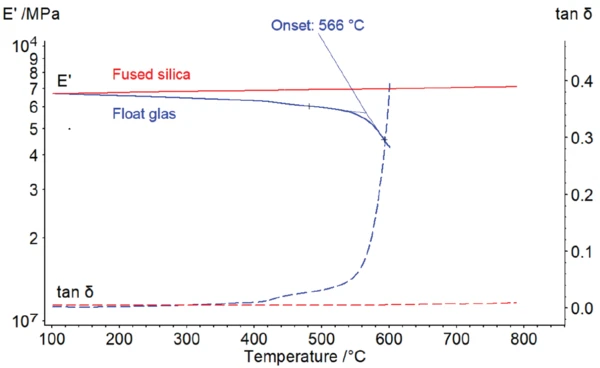
Both fused silica glass and pure silicate glass have a storage modulus, E', of just under 70 GPa at 100°C. The storage modulus, E', describes the elastic properties of the material; in simple terms, its stiffness.
With increasing temperature, the storage modulus of fused silica glass slightly decreases and takes on a value of approx. 60 GPa by 500°C. At 566°C (extrapolated onset), a strong decline in the storage modulus, E', occurs along with a significant increase in tan δ. Tan δ represents the damping properties of a material or its energy dissipation.
This is the Temperatura de Transição VítreaThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition (Tg) characteristic for amorphous solids. At temperatures below the Tg, materials are mostly solid and possibly brittle. In the Temperatura de Transição VítreaThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition, the kinetic energy of the unstructured atoms becomes sufficiently high to overcome intermediate bonding. At this point, the glass becomes softer and is shapeable. For this reason, the measurement is not continued upon reaching this point, in order to avoid melting of the glass at the sample holder.
In contrast, pure silicate glass, as shown in figure 1, exhibits behavior that is rather atypical for solids. Within the temperature range observed, softening of the material does not occur. Instead, the storage modulus, E', slightly increases with rising temperature. Babcock et al. [3] assume the co-existence of two atomic short-range order structures which have different bonding forces and densities. With increasing temperature, the structure with higher atomic bonding forces is increasingly formed and the material becomes stiffer.
The example demonstrates the use of pure silicate glass for high-temperature applications. While pure silicate glass can also be employed for temperatures above 600°C, conventional fused silica glass would no longer guarantee structural stability. Moreover, this example illustrates what different behavior can be exhibited by materials that are quite similar both visually and chemically, and how dynamic-mechanical analysis can help investigate this.
Summary
Dynamic-mechanical analysis is a method typically used to determine the glass transition of amorphous and semi-crystalline polymers. The DMA 303 Eplexor® allows for materials to be analyzed to 800°C – a temperature range that is unrivaled among bench-top instruments. This allows for even materials employed in the medium- to high-temperature range, such as metals, ceramics or glasses, to be characterized and evaluated for their application.