Introduction
The most widely used and economic flame redardant FR) for polymers is aluminum trihydroxide (Al(OH)3 or, abbreviated, ATH). It is used in plastics such as polyolefins for cable sheathing, but also in acrylics, thermoset resins and PVC flooring, to name some more applications. It is environmentally friendly as it contains no halogens and is highly efficient as a smoke suppressant.
Its flame retardancy* is due to cooling and barrier layer formation as well as dilution. The cooling capability stems from its ability to release water upon heating. The peak release occurs at around 300°C.
The underlying reaction is endothermic, which means the water consumes some of the released heat for evaporation.
The barrier functionality is a result of the Reação de DecomposiçãoA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of aluminum trihydroxide. The decomposed layer slows the flow of oxygen to the flame and thus the formation of gases. Large quantities (40-60 wt%) of filler must be used to obtain flame-retarding properties (dilution factor). As with most flame retardants (FRs), the addition of the filler also affects the mechanical and rheological properties of the plastics. Since the amounts of filler have to be high for its functionality, other additives have to be added to counteract their effect. Mechanical properties are improved by the morphology and surface coating of the Al(OH)3 to increase interfacial adhesion. The coatings differ depending on the base polymer to be used. The increased viscosity during processing gets counteracted by flow-enhancing additives.

Measurement Conditions
In this study, the effect of aluminum trihydroxide (ATH) on the fire behavior of polyethylene (PE) was investigated in the TCC 918 (figure 1). The instrument allows for the determination of the heat release, mass loss and density and composition of the smoke gas. To this end, samples of neat PE as well as PE with 50 wt% Al(OH)3 were injection-molded into 100 x 100 x 4-mm3 plates.
Before starting the tests, the gas analysis system (Siemens Oxymat/Ultramat) was calibrated with calibration gases and the C-factor was checked by using the methane burner with a defined heat release. The gas analyzer used was equipped with O2 and a CO2 option. After heating up the cone heater, the shutter was closed and the horizontal sample holder with the sample was mounted onto the ground plate. Then, the system automatically removed the shutter for the start of the measurement. The evaporated gases were ignited by the automatic ignition system. The measurement conditions are summarized in table 1.
Table 1: Measurement Conditions
| Sample holder | Horizontal |
| Heat-flux density | 50 kW/m² |
| Nominal dut flow rate | 24.0 l/s |
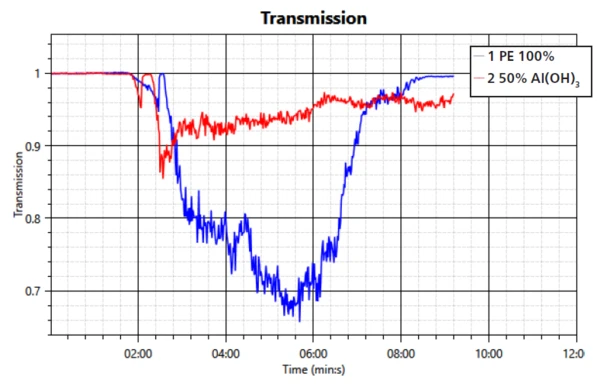
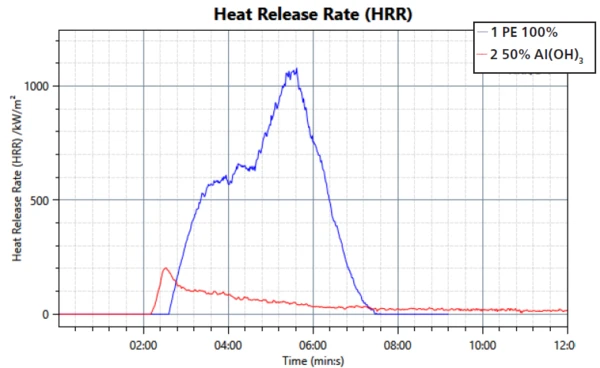
The heat release is in good agreement with the transmission measurement; see figure 3. The overall amount of released heat is smaller in the sample with the FR. However, the barrier function is again observable through a steady decrease in released heat.
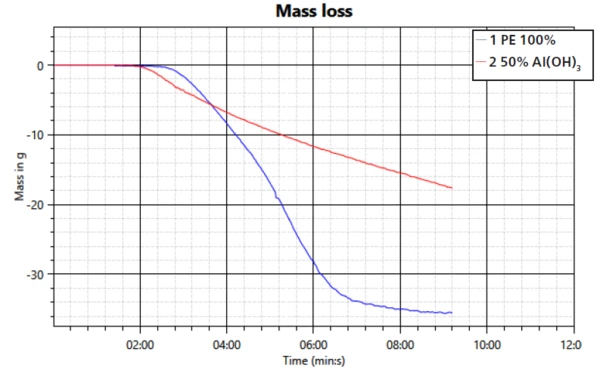
The mass loss accompanying the char formation is shown in figure 4. The mass loss occurs at a slower rate and to a lower degree. While the neat PE sample loses close to 35 g of weight by the end of the test, the sample with the flame retardant uses less than 20 g; only about half of it. However, it must be taken into consideration here that the sample with filler also contains only half PE.
Measurement in the cone calorimeter allows for study of the effect of a controlled fire exposure on a material; in this case, on a plastic with and without flame retardant. In this example, only the most important properties are depicted: transmission (smoke production), heat release and mass loss. However, it is possible in the same test to further analyze:
- Time of Ignition
- Mass-loss rate (MLR)
- Heat release rate (ARHE, MARHE)
- Effective heat of combustion (EHC)
- Total heat release (THR)
- Total smoke release (TSP)
- Smoke production (SPR)
- Combustion products
Conclusions
This study confirms the mechanisms of smoke suppression and formation of a barrier layer of the filler aluminum trihydroxide during a fire. The performance in regard to transmission property, heat release and mass loss was compared to a sample of PE without the flame retardant. It can be seen that the FR is working effectively when compounded into PE.