
31.05.2023 by Dr. Carolin Fischer
It is Barbeque Time! Choose For the Best Charcoal!
Summertime is barbecue time. But have you ever wondered which charcoal is best to use? The quality of charcoal can be characterized by the amount containing organic compounds, the ash content and energy released during combustion. These are all properties which can be determined using the simultaneous thermal analyzer NETZSCH STA. With the help of a TGA-DSC measurement, it is easy to check whether the price difference between products is justified by the quality.
For a comparison, three different kinds of commercial charcoal were selected: beech wood charcoal, brand-name charcoal and a cheap charcoal from a discount shop.
The TG-DSC measurements were performed with the simultaneous thermal analyzer NETZSCH STA equipped with a TG-DSC sample carrier type S. The different charcoal samples were heated as bulk samples to 550 °C in inert atmosphere and from 550 °C to 950 °C in oxidizing atmosphere. For detailed measurement conditions see table 1.
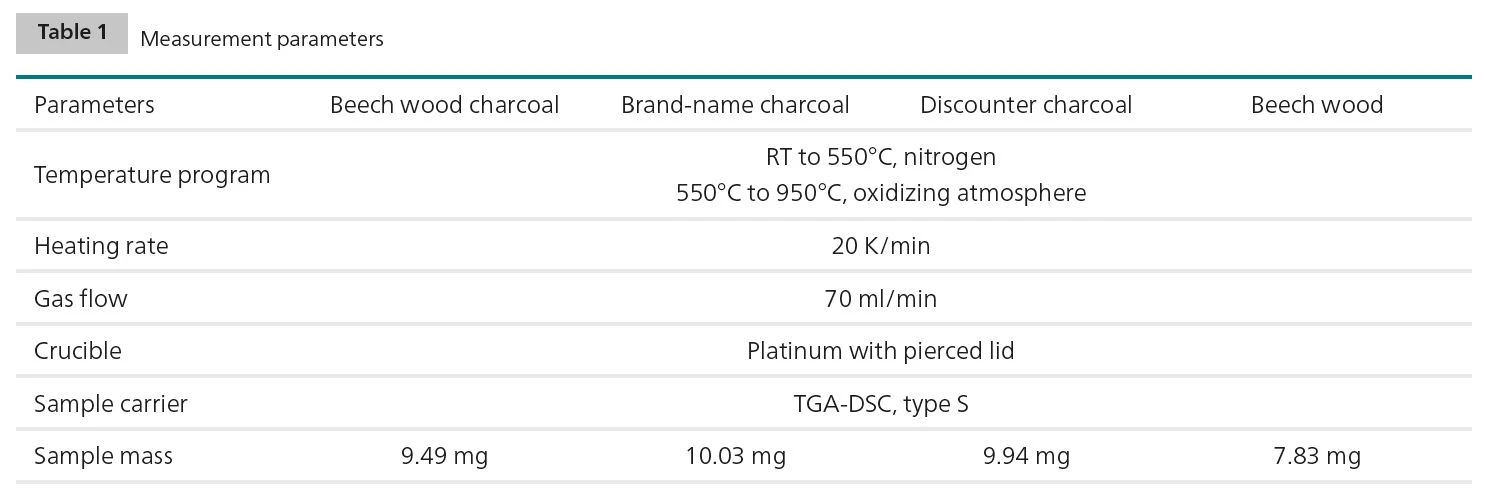
The results for the beech wood charcoal sample are plotted in figure 1. The three mass-loss steps were accompanied by energetic effects. The first mass loss step, at 81°C, was probably caused by the release of water, whereas the second mass loss, at 411°C, is an indication of the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of residual organic compounds. These events caused two endothermic effects at peak temperatures of 67°C and 394°C and enthalpies of 30 J/g and 5 J/g. The combustion of the remaining carbon under a synthetic air atmosphere resulted in a mass loss of 92% and an exothermic effect with an enthalpy of -23,315 J/g. This is not the complete combustion enthalpy, as an STA is an open system which emits part of the generated energy with the purge gases and the released gases. This value can only be used for relative comparison. The residual mass related to the ash content amounted to 3%.
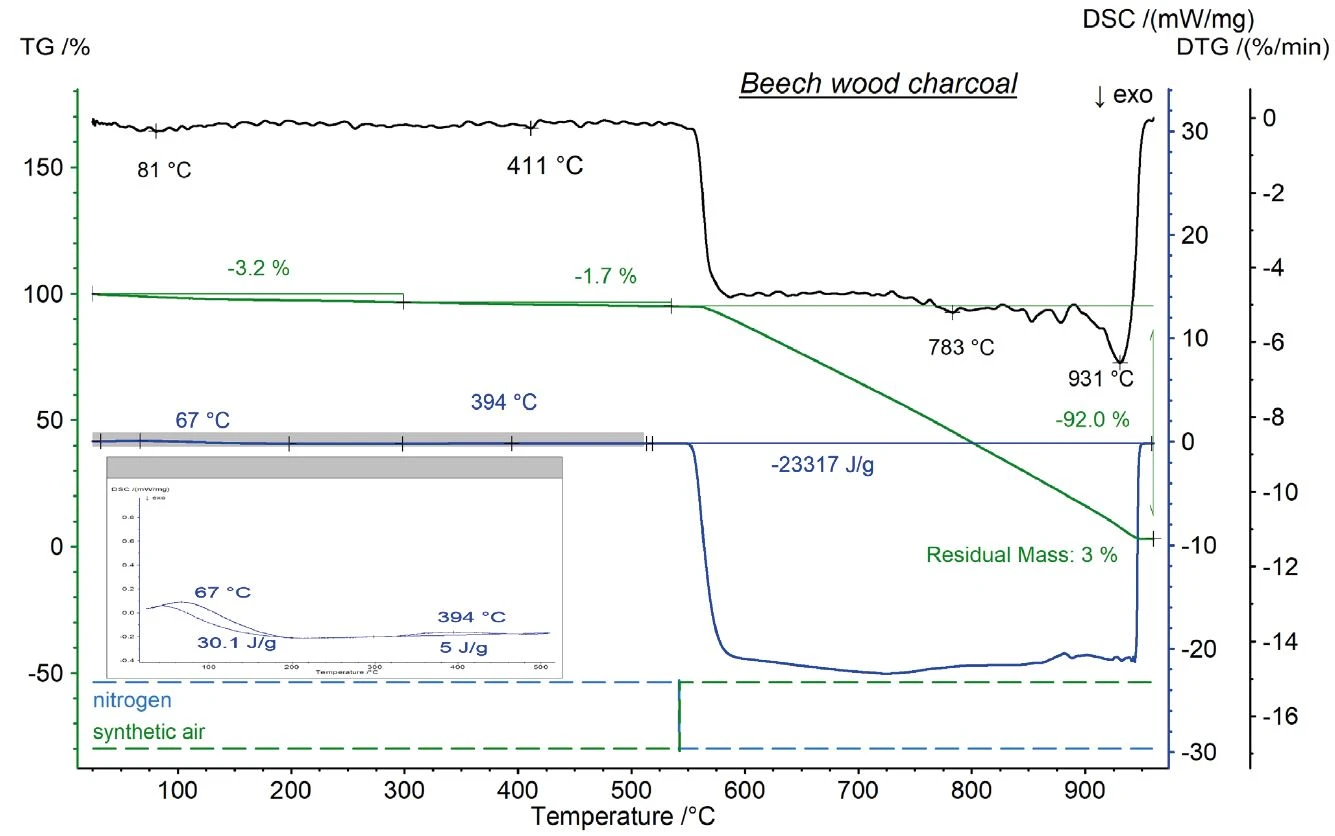
Figure 2 depicts the comparison of the TGA results for the different charcoal samples. The given temperature program led to two mass-loss steps for each sample under an inert atmosphere. In terms of water content, the brand-name charcoal showed the highest value, followed by the discounter charcoal and the beech wood charcoal. The differing water content is most probably due to different storage conditions, but may also be caused by differences in the surfaces’ properties which allow for the absorption of water.
In contrast, the proportion of organic compounds yields information about the level of completion of the production process for the charcoal and briquettes: The lower the organic content, the better the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of the initial wood to charcoal during the production process, yielding a higher quality charcoal. Comparing the three samples, again the beech wood charcoal exhibited the lowest value, followed by the brand-name charcoal and the discounter charcoal. This process was not yet finished at 550°C for the discounter charcoal, meaning that the sample still contains organic compounds at this temperature.
After switching to an oxidizing atmosphere, the residual carbon was burnt with oxygen and released carbon dioxide and carbon monoxide. Also here, differences between the three samples were observed. A carbon content of over 90% was determined for the beech wood charcoal, whereas both the brand-name charcoal and the discounter charcoal showed values of around 75% carbon. A high carbon content indicates high purity of the charcoal.
Consequently, the three samples also differ in terms of their residual mass, which characterizes the ash content of the charcoal. Surprisingly, the brand-name charcoal yielded more than 10% ash, whereas the other two showed values between 3% and 5%. The ash content can also be seen as a criterion of quality. The lower the ash content, the less the initial proportion of unreactive side products like fillers or minerals.
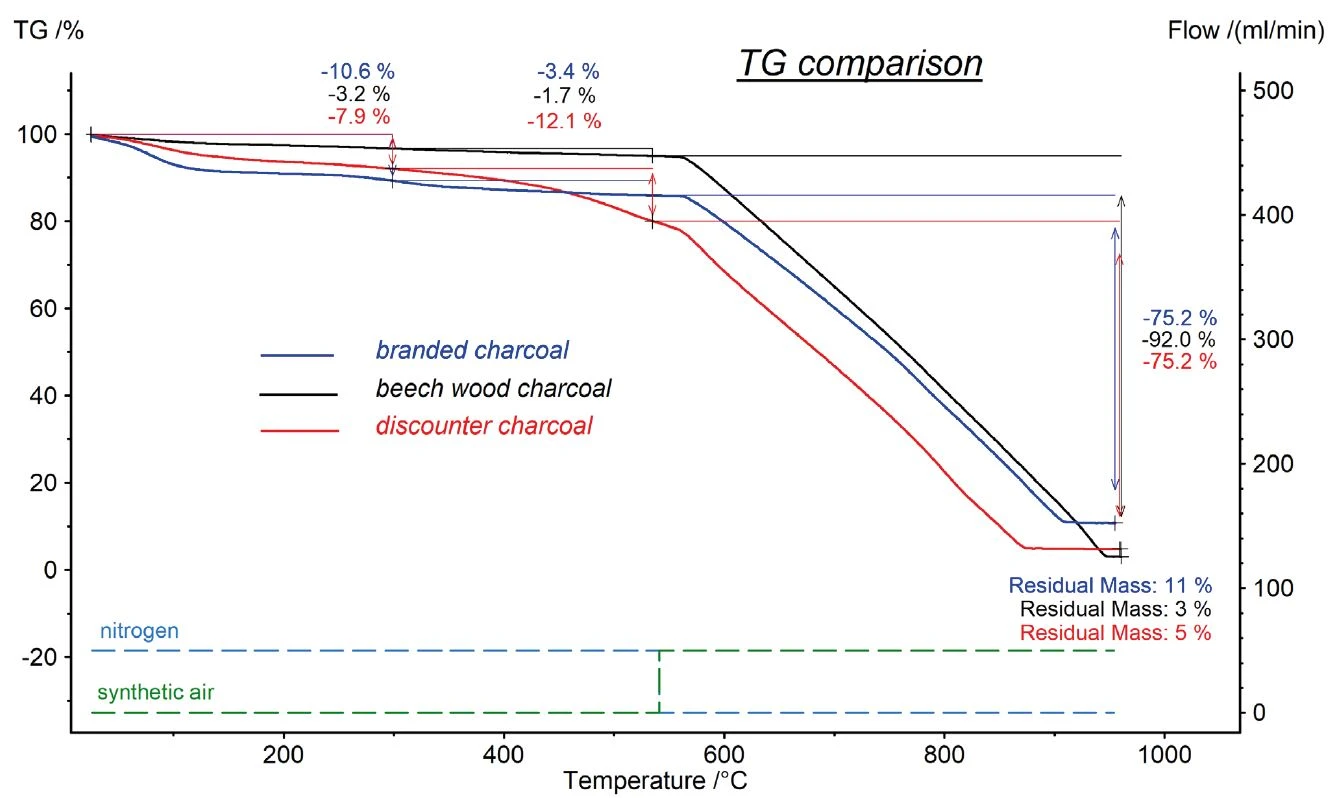
The comparison of the DSC signals, depicted in figure 3, showed that the beech wood charcoal released the most heat during the oxidative combustion. As the samples were measured in an open, non-AdiabáticoAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios
and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic system, these values cannot be regarded as the heat of combustion. The measured enthalpy is significantly lower than the heat of combustion, as hot reaction gases leave the sample and take the released heat with them. However, the released heat can be used as a good relative comparison between the three samples.
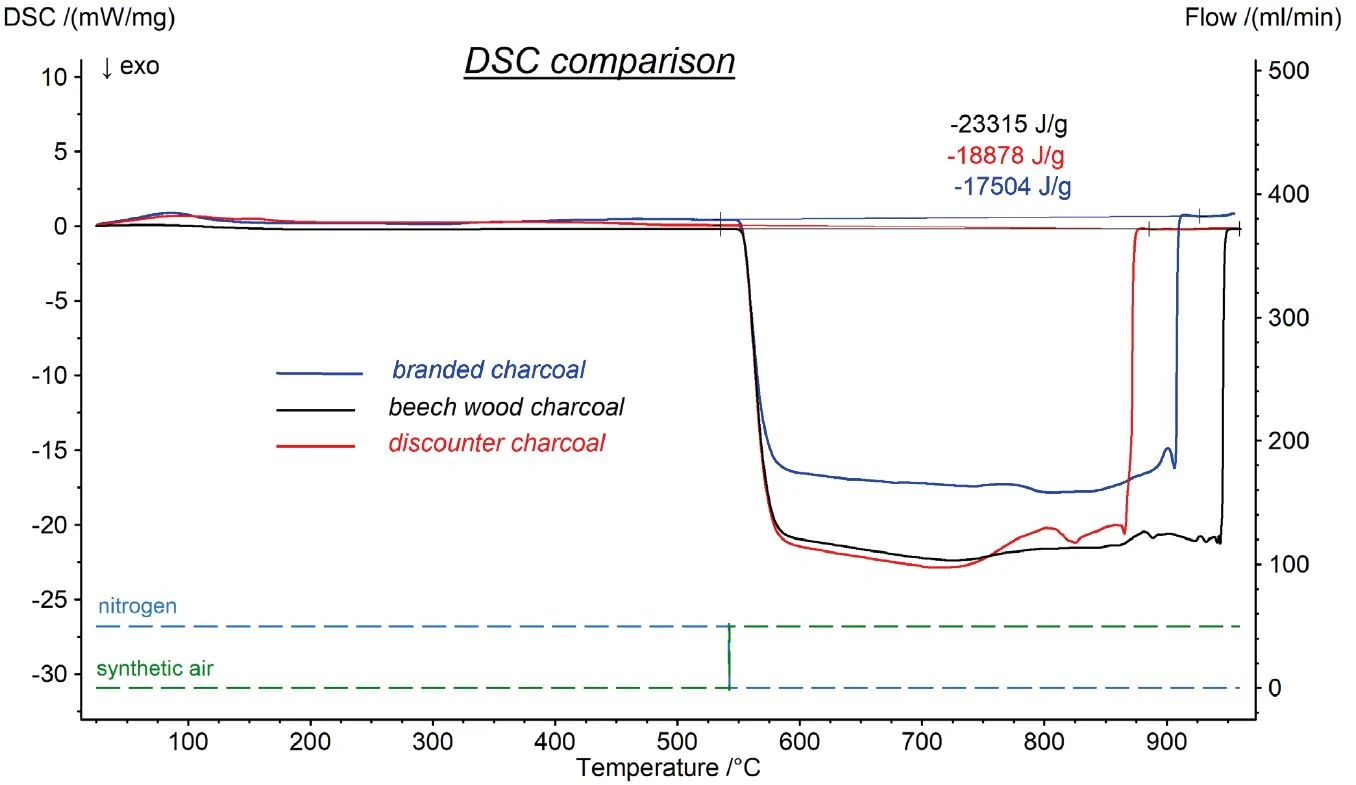
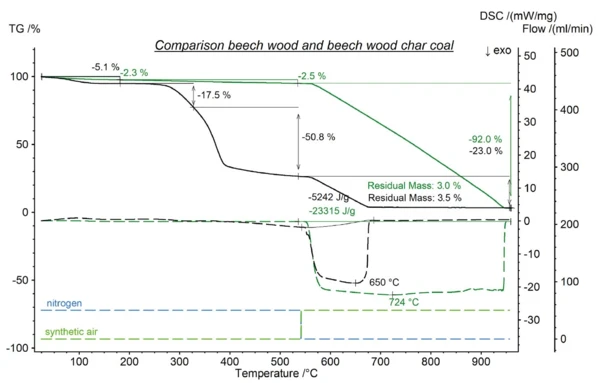
A further measurement was done with a beech wood sample, see figure 4. As expected, the amount of water and the organic content was much higher. The first the mass loss step, which refers to water, resulted in 5.13%. The increase of the temperature led to a two-stage Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the organic content amounting 68.35% in total. The comparison to the beech wood charcoal showed that the charcoal production process pyrolyzing the wood was nearly complete. The organic content was decreased from around 78% to less than 3%. The lower carbon content of the wood is also reflected in the exothermic enthalpy detected during oxidative combustion.
Summary
Quality characteristics of charcoals such as moisture, ash content and released heat can be detected with the help of the simultaneous thermal analyzer, STA, from NETZSCH Analyzing & Testing. It was possible to demonstrate the high quality of the beech wood charcoal with regard to these properties, whereas the brand-name charcoal did not show significantly better values than a discounter charcoal sample in this particular case. Additionally, the TG-DSC method is suitable to control the completion of the charcoal production process concerning the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of organic material.
We hope you enjoy your barbeque!

