14.05.2020 by Claire Strasser
Compressibility of Drug Products
Compressibility of a pharmaceutical substance can lead to changes in its crystal structure, and thus in its properties (bio-availability, flowability, …). A DSC measurement verifies the quality of the product after compression.
Compactibility is the ability of a powder to form a mechanically strong tablet, whereas compressibility is the ability of a powder to be compressed and consequently be reduced in volume [1]. The manufacture of tablets requires a well compressible API (Active Pharmaceutical Ingredient) or its combination with adequate excipients responsible for the good compressibility behavior of the formulation. In this case, a main challenge is to find the excipients that don't affect the properties, stability, efficacy of the active ingredient (please click here to get more information about Drug-Excipient CompatibilityMedicine is not a single active ingredient but a mixture of the active ingredient with different excipients. In a tablet for example, excipients are used to improve the appearance and taste of the final product, to prevent the tablet from sticking to the punching tool, or to help it dissolve as soon as it is wet, etc.drug-excipient compatibility). Some elements can influence the compressibility behavior as well as the properties of the compact:
- Humidity of the environment storage;
- Particle size;
- Polymorphism.
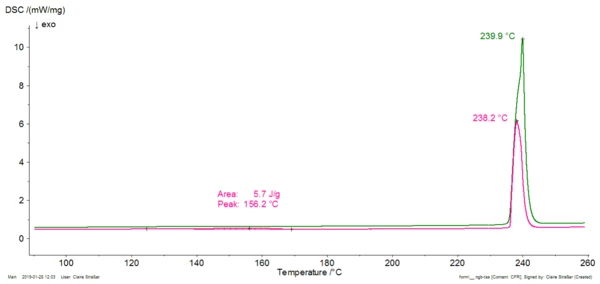
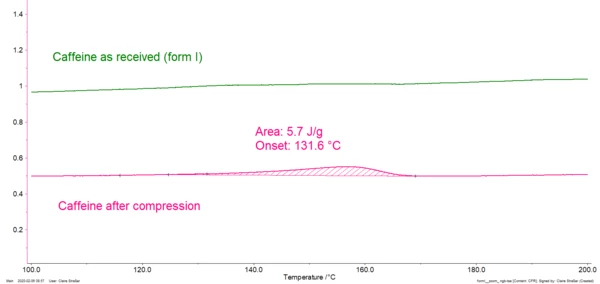
Compression of a polymorphic pharmaceutical product can lead to a crystal modification, and thus to changes in its properties (bio-availability, flowability, …). Differential Scanning Calorimetry (DSC) is an easy and fast method to ensure that the compression process doesn't influence the crystal structure. Let us take the example of caffeine. This substance used in association with active ingredients that induce sleepiness exists under different modifications called forms I and II. Both modifications exhibit different mechanical properties. Figure 1 depicts the DSC curves of caffeine as received (green curve) and after 20 minutes compression with 20 kN followed by a one-week storage at room temperature (pink curve). The green curve is typical for the modification I of caffeine (melting peak at 240°C). The pink curve additionally exhibits an endothermal peak at 156°C resulting from the transformation of form II into form I (see zoom, bottom plot).
Figure 1. DSC measurement on caffeine before compression (green curve) and after 20 minutes under 20 kN followed by a 1-week-storage at room temperature. The peak beginning at 156°C (zoom in bottom plot, extrapolated onset temperature) is typical for the transformation of modification II into modification I. This shows that caffeine changed its modification during compression and therefore its properties. In particular, a powder mixture made of microcrystalline cellulose and caffeine II has a better ability to deform under compression than if caffeine II is replaced with caffeine I [2]. You will get detailed information on this example here.
Sources:
[1] The Compaction of Pharmaceutical Powders, Oluwatoyin A. Odeku, Pharmaceutical Reviews · January 2007 [2] Sébastien Hubert. Transitions de phases solides induites par un procédé de compression directe : application à la caféine et à la carbamazépine. Alimentation et Nutrition. Université Claude Bernard – Lyon I, 2012.