
03.08.2021 by Dr. Gabriele Kaiser
Magnesium Stearate – One Material, Different Compositions easily Distinguishable by DSC
Magnesium stearate is one of the most frequently used pharmaceutical excipients. Learn more about its hydration state and how it can be made visible.
Magnesium stearate, with the abbreviation MgSt, is one of the most frequently used pharmaceutical excipients. Some years ago, the lubricant could be found in more than half of the top 200 pharmaceutical formulations on the market [1].
Although pharmaceutical lubricants are added to solid dosage forms only in a small quantity (typically in a proportion of 0.2 to 5 wt %), they can – amongst other things – effectively decrease the friction between the compacted tablet and the die during tableting [1]. Therefore, they play an important role in the manufacturing process.
However, despite its popularity, magnesium stearate is not very well defined. In the US pharmacopeia ([2]), it is described as a compound of magnesium with a mixture of solid organic acids, which mainly consists of magnesium stearate and magnesium palmitate in variable proportions. As a result, many commercial grades of magnesium stearate do not correspond to the formula (MgC36H70O4) and molecular mass (591.2 g/mol) of pure magnesium stearate reported in literature.
Pseudo-Polymorphism and its Effect on Material Properties
Magnesium stearate exists in its anhydrous form, as an ordered monohydrate, a disordered monohydrate, a dihydrate and a trihydrate [1]. Depending on temperature and relative humidity, interconversion can take place between these hydrates [3]. Therefore, many commercial types of magnesium stearate are not composed of one hydrate only but of a mixture of them [4].
However, the lubrication efficiency and tabletability of magnesium stearate is linked to the physicochemical properties of the material and thus also to its hydration state. [5] Hence, it is relevant to select magnesium stearate, which meets the requirements for the particular formulation, and to maintain this quality consistently. Here, DSC is of great help.
Differentiation at a Glance
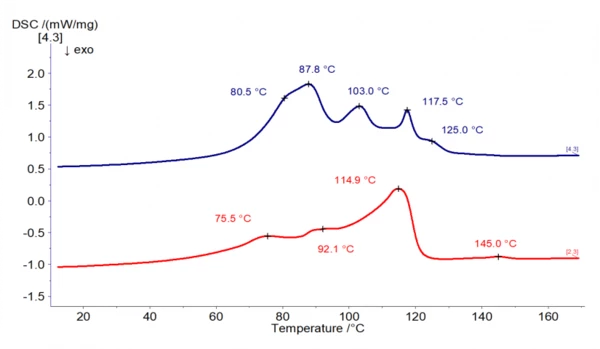
Figure 1 illustrates the first heatings obtained during the DSC measurements on two commercial magnesium stearate types for pharmaceutical use. The visible thermal effects reflect dehydration (as well as melting) of the sample. TGA-FT-IR investigations (not shown here) have verified that really only water is released in the given temperature range up to 170°C.
The complex DSC curve shapes suggest that we are dealing with a mixture of hydrates in both cases. However, the curves indicate that the water is located at quite different positions. This is a clear sign that the sample materials do not have the same hydration state.
References:
[1] S.P. Delaney et al., Characterization of Synthesized and Commercial Forms of Magnesium Stearate Using Differential Scanning Calorimetry, Thermogravimetric Analysis, Powder X-Ray Diffraction, and Solid-State NMR Spectroscopy, J. Pharm. Sci. 106 (2017), 338 – 347
[2] Monograph text about Magnesium Stearate in USP, Stage 6 Harmonization, Official August 1, 2016
[3] S.H. Wu, Effect of Magnesium Stearate Lubricant Attributes on Product Processibility and Quality, presented in 2009 Land O´Lake Industrial Pharmacy Conference, available as slide share presentation under Attributes of Magesium Stearate as a Tablet Lubricant (slideshare.net)
[4] J. Li and Y. Wu, Lubricants in Pharmaceutical Solid Dosage Forms, Lubricants(2014), 2, 21-43; doi:10.3390/lubricants2010021
[5] J.L. Calahan, Correlating the Physicochemical Properties of Magnesium Stearate with Tablet Dissolution and Lubrication, University of Kentucky, Theses and Dissertations–Pharmacy. 117