07.02.2024 by Dr. Elena Moukhina, Aileen Sammler
Introducing Termica Neo: The New NETZSCH Software for Thermal Simulation in Industrial Settings
The new Termica Neo software enables users not only to analyze and simulate laboratory processes easily and precisely, but also to predict high-volume (kilograms and tons!) industrial processes to maintain the best product quality and safety.

For the simulation of temperature-dependent processes in the chemical industry, the temperature gradients in the reacting media could be significant and must be taken into consideration. For processes like Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing or CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization, the temperature gradient has an influence on product quality, and for highly ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal material it has an influence on the safety conditions of runaway reactions.
We are proud to present our new NETZSCH Termica Neo software, which uses all kinetic methods recommended by ICTAC*[1], is completely compatible with the NETZSCH-software Kinetics Neo, and works for both model-free and model-based approaches as well as for complex reactions with independent, competing, or consecutive steps.
*ICTAC: International Confederation of National or Regional Thermal Analysis and Calorimetry Societies. The aim of ICTAC is to promote international understanding and cooperation in thermal analysis and calorimetry through the organization of international congresses and the work of its scientific committees. (See also ictac.org)
[1] Vyazovkin S et al, ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics, Thermochimica Acta, V.689, 2020, 178597
Simulation of High-Scale Industrial Processes to Avoid Runaway Actions and Explosion
Small samples with a weight of only a few milligrams measured by means of Differential Scanning Calorimetry (DSC) or with other thermal analytical methods like Thermogravimetric Analysis (TGA) or Accelerating Rate Calorimetry (ARC®) have no significant temperature gradient and are therefore suitable for kinetic analysis. The kinetic software can simulate the rate of chemical reactions for two limiting cases where samples have no temperature gradient. In the first case, the sample material has both infinite Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity and infinite heat transfer to the surrounding, within a controlled surrounding. The second limiting case is pure AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC®). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic heating without any heat loss.
However, in the chemical industry, as well as in the storage and transport of highly energetic materials, heat transport and heat loss are between these two limiting cases; and for safe conditions or to achieve desired product quality, we need to carry out the simulation for non-constant temperature in reacting volume.
The main applications of such a simulation in industry are product quality and safety.
In the polymer or ceramic industry, the areas with higher temperature have a higher reaction rate, which leads to differing physical properties of material at different coordinate points. This appears as shrinkage during SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering or Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing, which produces mechanical StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress and has an influence on product quality.
In predictions regarding the storage or transportation of highly energetic material in the chemical industry, the temperature gradients in the reacting media are also significant and must be taken into consideration. For highly ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal reactions, the areas with higher temperature and faster reactions have more intensive heat production and self-heating. Then such local areas become the hotspots for the beginning of runaway or thermal explosion. For reactions with lower thermal effect, the areas with higher temperature have a higher reaction rate and degree of conversion. This is the reason for the differing physical and chemical properties of materials at different coordinate points, which appear as heat capacity, heat conductivity or concentrations of reactants.
Simulation of Complex Chemical Processes with Termica Neo
Many existing FEM (Finite Element Method) software solutions can calculate the heat transfer, but they are limited in terms of complex multi-step chemical reactions with thermal effects present. Usually such systems work for model-free kinetics with a single kinetic equation, or for models with 1-2 steps in which all kinetic parameters are known.
The new Termica Neo software for thermal simulation consists of input data in the form of chemical parameters and equations directly from Kinetics Neo project. It is completely compatible with NETZSCH Kinetics Neo Software and is able to use both model-free and model-based approaches. For the model-based approach, there are no limitations on the number of individual reaction steps or on connections between them, including independent, competing, or consecutive ones.
The Termica Neo simulation software accepts all kinetic parameters from Kinetics Neo. It additionally uses temperature-dependent physical parameters like DensityThe mass density is defined as the ratio between mass and volume. density, Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, and the heat capacity of reacting materials and products from the materials library. The additional input parameters include containers for which the thickness and material can differ for each surface of reactor geometry and also include different surrounding media, for example, air on the top, water on the side and ground on the bottom. The surrounding temperature profiles can also be different for different geometry surfaces.
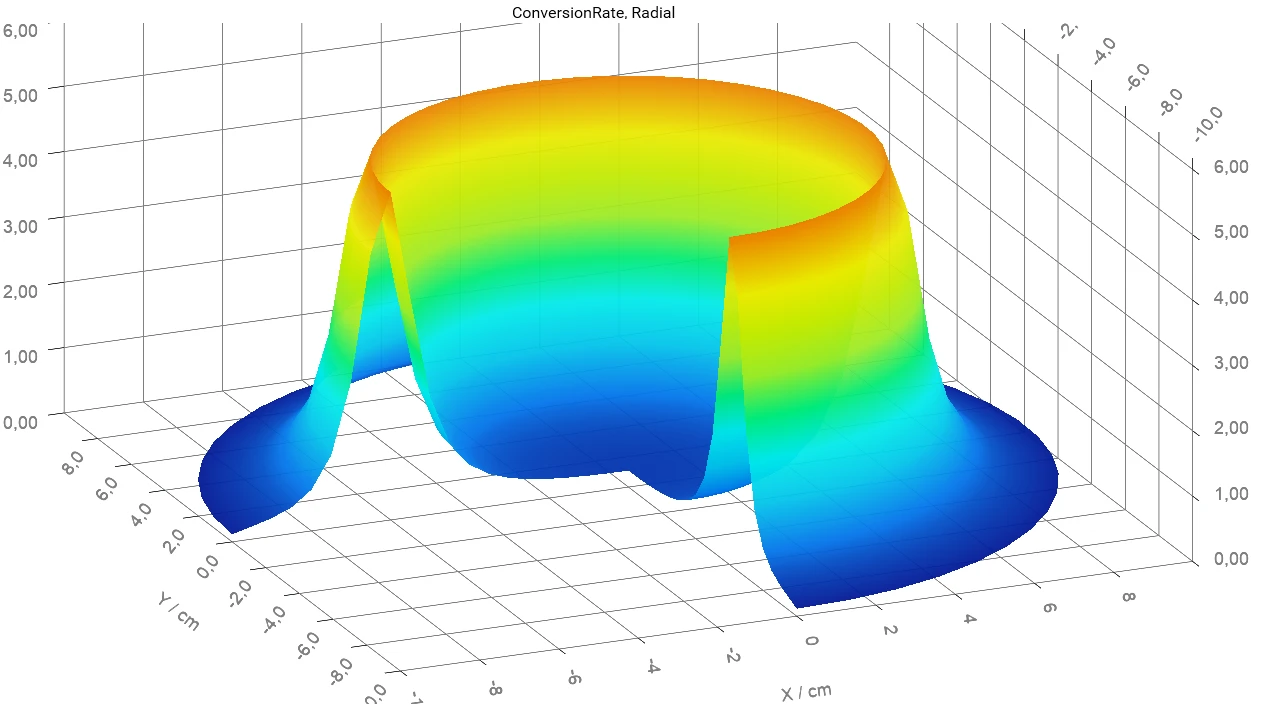
What You can Do with Termica Neo
- Simulate your materials’ behavior at each point inside the container
- Find out where and when the maximum temperature or maximum conversion rate of the reactant inside the container are
- Determine the temperature, conversion, and concentrations for a given time and position of the reactant inside the container
- Predict your degree of Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition, and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization
- Determine thermal safety conditions for production and storage
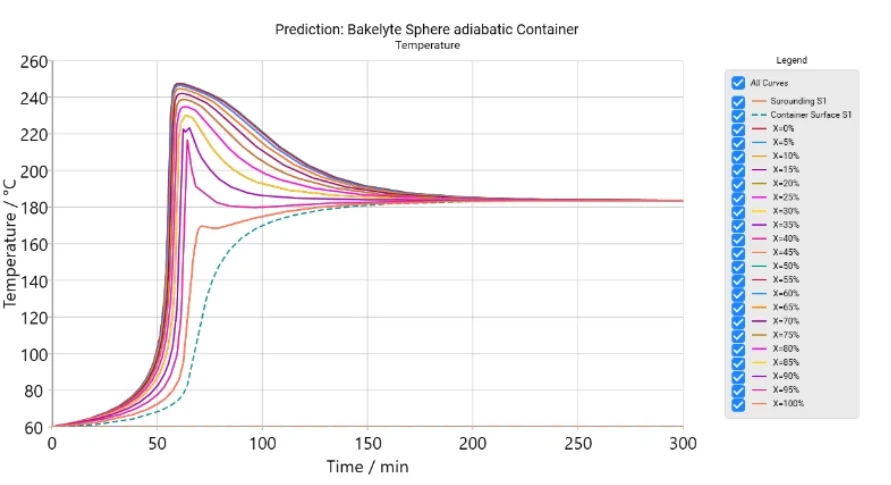
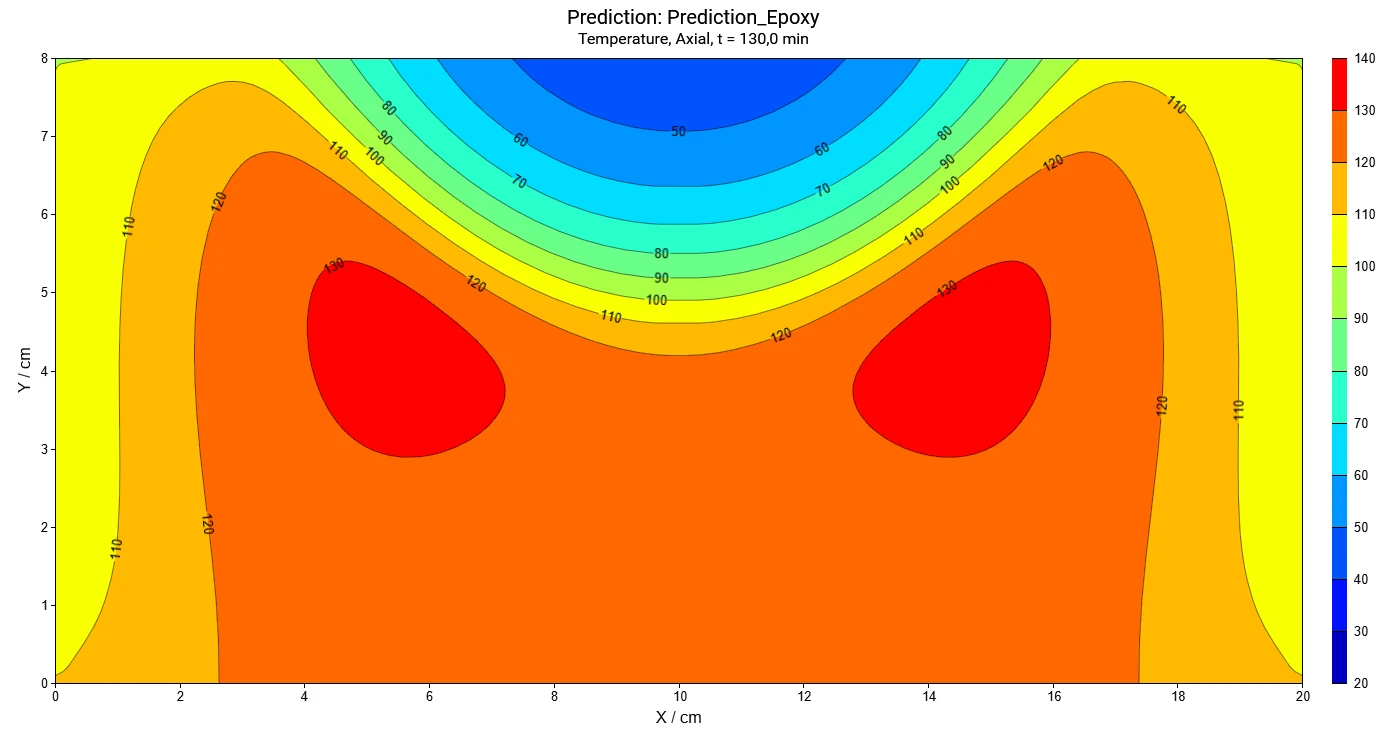
The software provides time-dependent and coordinate-dependent results for temperature, concentrations of all reactants, and reaction rates in 2D and 3D view. A search for self-accelerating Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperature (SADT) as well as a simulation of AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC®). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic conditions and infinite heat transfer to the surrounding are also available.
Features at a Glance:
- Fast and easy-to-handle: User interface similar to Kinetics Neo software
- The kinetic models are taken directly from the Kinetics Neo project (results for any method including both model-based and model-free).
- Calculation of the following properties at each point in volume as a function of time:
- temperature,
- conversion,
- conversion rate,
- concentrations,
- Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature for reactions of Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing or cross-linking
- Calculation of Self-Accelerating Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. Decomposition Temperature (SADT) under various materials, containers and surroundings.
- Simulation of reactions for a reactor with container including AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC®). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic conditions