Introduction
In the last few years, the nail polish industry has really opened up its beauty box of tricks and on the market today. To name but a few, we see nail polishes with “magnetic, gel, matte, glitter, crackle, stamping, quickdry, caviar, sponge, colour changing, leather and sandy” effects. There are also nail polishes with kale, silk and nylon extracts! It really has turned into an art form.
However, in order for us to appreciate the wonders of these magical nail polishes, surely there must be some exciting science behind it all? Many may have wondered and are fascinated by these different effects such as why “stamps”, why crackle polish “crackles” and why UV nail polish “cures”, etc.
Different Types of Gel Polishes Cure Differently
Believe it or not, there are varying degrees of Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing gel polishes; those that are displayed in your local retailer as ‘daylight Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing’ polish, those ‘hybrid’ gels that can be bought from your local shopping centre and then, of course, those ‘soak-off’ gels found only in your local beauty salon! Both the hybrid and soakoff gels require the use of a “UV lamp” to cure. The difference being that the hybrid gels incorporate similar solvents and additives as regular nail polish, allowing them to soak off faster and possess a lower viscosity for easier application. Traditional gels have a higher degree of cross-linking, resulting in a greater resistance to acetone [1].
How to Study Curing Behavior
The Kinexus rheometer was used to study different colored UV-Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing nail gels (of the soak-off variety) in the shades of gold glitter, red, pink and black. By applying a fixed intensity of UV light to the gels for 30 seconds, it is possible to monitor the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing profile and consequently the change in modulus (stiffness) in these materials over time. Distinct differences were seen between gels with different pigments (see figure 1). The clear gel with gold glitter particles cured the fastest of all four gels, exhibiting a much higher modulus (~7.5 · 107 Pa) in comparison to the black gel, which cured slower resulting in a much lower shear modulus (~3.7 · 105 Pa).
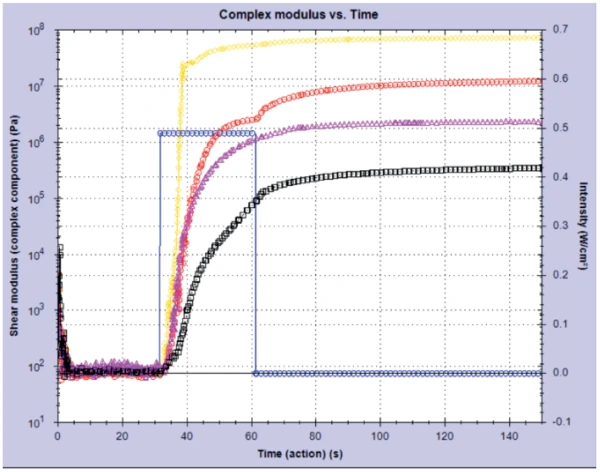
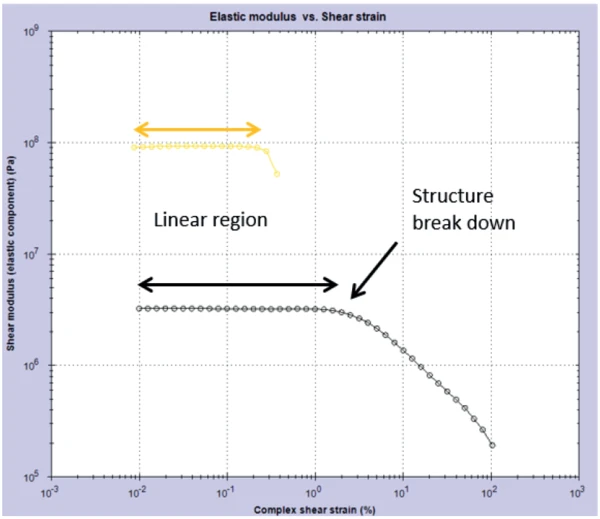
This result was confirmed by conducting a post-cure amplitude sweep on the gels. This measurement probed the linear visco-elastic region (Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.LVER) of the samples by applying an increasing StrainStrain describes a deformation of a material, which is loaded mechanically by an external force or stress. Rubber compounds show creep properties, if a static load is applied.strain and determining the point at which the structure in the material was broken down – the onset of non-linearity. Figure 2 shows the results from this experiment and it can be clearly seen that the black gel has a longer Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.LVER than the glitter gel.
Summary
Based on the results, it can be said that the black gel will be more flexible on the nail and is more likely to be easily removed. Due to the smaller (in StrainStrain describes a deformation of a material, which is loaded mechanically by an external force or stress. Rubber compounds show creep properties, if a static load is applied.strain magnitude) Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.linear viscoelastic region, the glitter gel will exhibit more brittle properties and may also be harder to remove. It is instances like these where rheology can be employed as a useful tool in determining differences and characterizing products that will be noticeable and important to consumers.
This application note has focused on Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing nail polish and the influence of pigments. However, the rheology of nail polishes builds up a picture of how the material behaves in use. Just like paints, nail polishes exhibit thixotropic properties – the properties that most importantly give you that nice smooth and even surface finish.