
06.02.2020 by Milena Riedl
Good Things Come to Those who Wait … That was in the Past!
Most industrial chemical processes are based on catalysis, which means that its economic importance is enormous. NETZSCH, Micromeritics and Malvern Panalytical are jointly organizing a two and a half day workshop on analytical techniques and methods for the characterization and optimization of heterogeneous catalysts in March 2020. Learn more about the program in this blog article!
by Dr. Michael Schöneich, Applications Laboratory, NETZSCH-Gerätebau GmbH, Germany
Even before the discovery of the phenomenon of catalysis by Berzelius in 1835, mankind had already been using catalysis extensively for centuries. Just think of the fermentation during beer brewing or the production of vinegar by the chemical formula known today: ethanol + oxygen → acetic acid + water. However, this formula was not known before, so the conversion was random. It could take more than six months for the bacteria to ferment the alcoholic liquid. In addition, not every conversion attempt was successful (unfortunately, the air contains more than just the vinegar bacteria). In the 16th and 17th centuries, catalytic reactions also included, for example, soaps by fat hydrolysis and diethyl ether by dehydration of ethanol. In 1895, Ostwald described catalysis as an acceleration of chemical reactions through the presence of foreign substances which are not consumed; he said that “a catalyst is a substance that changes the rate but not the thermodynamics of a chemical reaction”. In 1909, he was awarded the Nobel Prize. Illustratively, a catalyst can be compared with a “gate keeper”. The catalyst opens a gate or a barrier. This allows for a shorter and faster route that does not lead over the top or peak and therefore requires less energy. Since the catalyst is only a “gate keeper”, nothing happens to it.
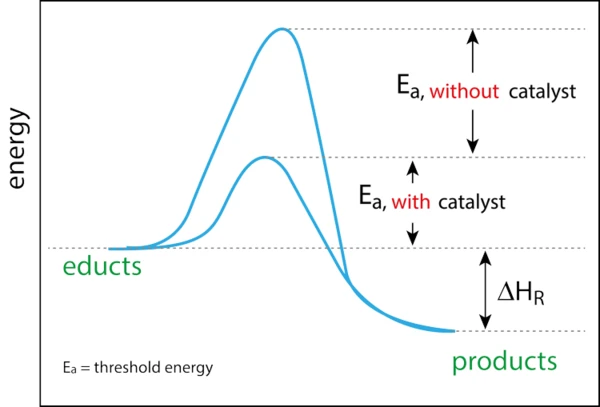
There are two types of catalysts, homogeneous and heterogeneous. A homogeneous catalyst is a catalyst that is in the same phase as the reactants, while a heterogeneous catalyst is in a different phase than the reactants. Today, most industrial chemical processes are based on catalysis, which means that its economic importance is enormous. More than 80% of the industrial processes established for 40 years in the chemical, petrochemical and biochemical industries as well as in polymer production and environmental protection use catalysts. Catalysis enables lower energy processes, less waste and pollution and improved selectivity in the manufacture of products with added value for all sectors. Heterogeneous catalysts are already a key component of this sector, e.g. petrochemical conversions, and offer significant benefits such as re-use and recyclability. As the world moves towards more sustainable technologies and raw materials to ensure a cleaner future, heterogeneous catalysts will play an even greater role.
Characterization Workshop for Heterogeneous Catalysts
NETZSCH, Micromeritics and Malvern Panalytical are jointly organizing a two and a half day workshop on analytical techniques and methods for the characterization and optimization of heterogeneous catalysts at the premises of Micromeritics in Unterschleissheim near Munich between March 16 and 18, 2020. The workshop (English language) is targeted at all people working in the field of Heterogeneous Catalysts including fundamental research, production and end use applications, and will cover the following topics:
- Physisorption / Chemisorption
- Thermal Analysis
- Particle Characterization
- Structural and Elemental Analysis
The workshop will also include practical sessions where delegates will see the various techniques being demonstrated by a team of application specialists. Interested?
Click here to see the full program and register for the workshop!