Glossary
Carbon Black
Temperature and atmosphere (purge gas) affect the mass change results. By changing the atmosphere from, e.g., nitrogen to air during the TGA measurement, separation and quantification of additives, e.g., carbon black, and the bulk polymer can become possible.
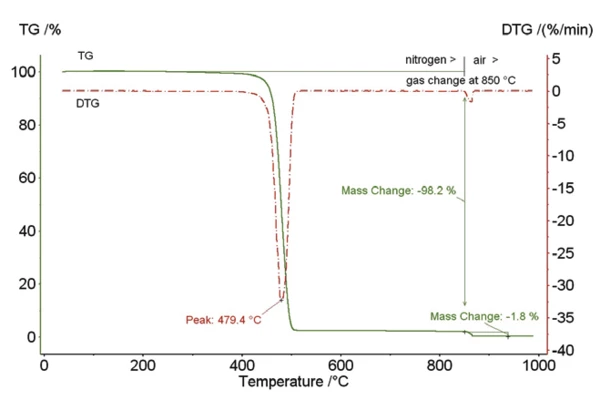
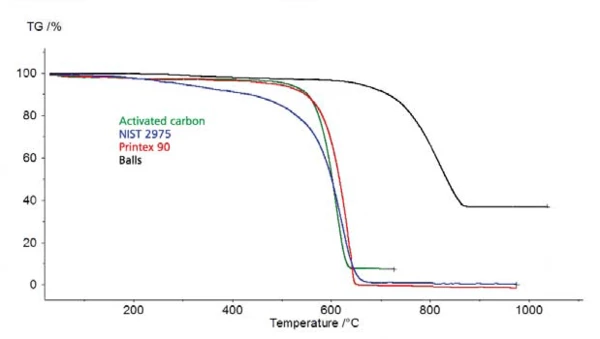

Figure 1
Figure 1 shows polyethylene (PE) filled with 1.8% carbon black. The first Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition step (DTG peak at 479°C) refers to the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of PE. After switching from an inert gas atmosphere (nitrogen) to an oxidizing atmosphere (synthetic air), the Added Carbon BlackCarbon black acts as a reinforcing filler in tires and other rubber products. In other materials such as plastics, paints, and inks, carbon black is used as a color pigment or as filler to achieve electric conductivity.added carbon black totally burns to carbon dioxide.
Figure 2
The surface of the carbon black determines the combustion behavior (in oxygen atmosphere). The larger the surface area, the smaller the particle size, the lower the combustion temperature or the faster the combustion takes place at a defined temperature (see figure 2).
Due to this combustion behavior, it is in many cases possible to distinguish between Added Carbon BlackCarbon black acts as a reinforcing filler in tires and other rubber products. In other materials such as plastics, paints, and inks, carbon black is used as a color pigment or as filler to achieve electric conductivity.added carbon black and Pyrolytic CarbonPyrolytic carbon is carbon which is generated by the pyrolysis of organic matter in an oxygen-free atmosphere. pyrolytic carbon. Therefore, the TGA can be used to determine the carbon black content – even in polymers which form pyrolytic soot.


