Glossary
Solid-Solid Transitions
Solid-solid transitions are observed in single-component systems, but also in multi-component ones. By changing the temperature (or pressure), a crystalline structure can be transformed into another crystalline structure without entering an isotropic liquid phase.
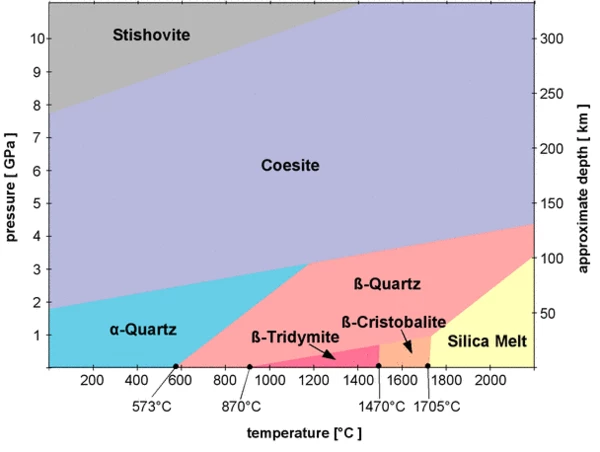
These transitions result in material polymorphs. In most cases, crystalline solid-solid transitions are first-order transitions which undergo discontinuous changes in volume, enthalpy, and entropy due to crystal, lattice or packing changes. The magnitude of these changes is usually small compared to the changes caused by crystalline solid-liquid transitions. For example, a well-known solid-solid Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition is the α→β transition of SiO2, also called quartz, at 573°C (standard pressure).
Along with DSC, the combined TGA-DSC method is a powerful tool for investigating such kinds of transitions. Furthermore, dilatometry is also a very useful tool for characterizing the type of solid-solid transitions as mentioned.