Introduction
In the molten state, the polymer chains of a semi-crystalline polymer are in a disordered state. During cooling, some of them rearrange to form ordered regions and crystallize. In addition to this crystalline phase, a semicrystalline polymer also contains an amorphous phase without an ordered molecular structure (see figure 1). Cooling does not lead to CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of this phase, but to a transitions from a soft to a hard brittle state. This transition is called the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition.
Different methods can characterize the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization and Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polymers, providing a variety of valuable information.
A typical method for analyzing thermal transitions is Differential Scanning Calorimetry (DSC). It provides information about the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition, phase transformations like CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization/melting or solid-solid Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transitions and degree of Crystallinity / Degree of CrystallinityCrystallinity refers to the degree of structural order of a solid. In a crystal, the arrangement of atoms or molecules is consistent and repetitive. Many materials such as glass ceramics and some polymers can be prepared in such a way as to produce a mixture of crystalline and amorphous regions.crystallinity, etc. Its ease-of-use and ability to automate measurement steps have made it a popular and widely used technique.
CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization and Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition have a significant influence on the mechanical properties of a product. Another method for determining these parameters is rheology. A measurement using a rotational rheometer provides information on the rheological changes that occur as a semi-crystalline polymer cools from the melt into the glassy state. In the following, the cooling behavior of polyether ether ketone (PEEK) (see chemical structure in figure 2) is determined using the DSC 303 Caliris® and Kinexus rotational rheometer.


Measurement Parameters
The PEEK sample was heated to above its Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature. After an IsothermalTests at controlled and constant temperature are called isothermal.isothermal phase, the polymer was cooled down at a controlled cooling rate. The standard cooling rates of the respective methods were used, i.e., 10 K/min for the DSC 300 Caliris® and 2 K/min for the Kinexus rotational rheometer. Table 1 summarizes the measurement conditions.
Table 1: Measurement parameters
| Instrument | DSC 300 Caliris® | Kinexus HTC Prime |
| Crucible | Concavus® (aluminum) | - |
| Sample mass | 9.80 mg | - |
| Temperature program | 370° to 30°C | 400°C to 40°C |
| Cooling rate | 10 K/min | 2 K/min |
| Atmosphere | Nitrogen (40 ml/min) | NItrogen (1 ml/min) |
| Geometry | - | PP8 (plate-plate, diameter: 8 mm) |
| Gap | - | 1 mm |
| Shear StrainStrain describes a deformation of a material, which is loaded mechanically by an external force or stress. Rubber compounds show creep properties, if a static load is applied.strain | - | Within linear-viscoelastic range (Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.LVER) |
| Frequency | - | 1 Hz |
DSC 300 Caliris®: Crystallization Behavior
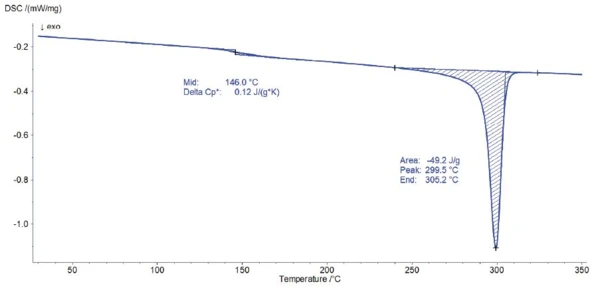
Figure 3 displays the resulting curve of the DSC measurement performed on PEEK. The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak beginning at 305°C (endset temperature) is due to the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of PEEK. The step in the DSC curve with midpoint at 146°C is the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition.
Kinexus Rotational Rheometer: Stiffness
Figures 4 and 5 depict the typical curves resulting from the temperature sweep performed on PEEK.


The Melt State
Providing no reaction occurs, the complex shear viscosity (figure 4) increases with decreasing temperature. This is the expected influence of temperature on stiffness in the absence of a physical or chemical process, as the mobility of polymer chains increases during heating.
The melt state is also characterized by domination of G” over G´ (figure 5). In other words, at this temperature, the “liquid-like” properties have more influence on the deformation behavior of PEEK than the “solid-like” properties. The polymer flows for the timescale of the applied frequency, even if it still features strong elastic properties (phase angle value closer to the value 45° than to 90°).
At 325°C, the slope of the complex shear viscosity curve changes (Figure 4). The complex shear viscosity increases from 7.7E+03 Pa∙s at 325°C to 9.0E+06 Pa∙s at 295°C, an increase of more than 3 decades in only 30°C! This significant increase is typical for the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of a crystalline or semi-crystalline polymer.
The process also greatly affects the elastic (G') and viscous (G") shear moduli (figure 5). Both curves increase and show the crossover at 308°C. BetweenCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization and glass transition, the amorphous phase is in the rubbery plateau. The polymer chains belonging to the amorphous phase are still free to move, while the crystalline phase gives structure to the product.
The higher the degree of Crystallinity / Degree of CrystallinityCrystallinity refers to the degree of structural order of a solid. In a crystal, the arrangement of atoms or molecules is consistent and repetitive. Many materials such as glass ceramics and some polymers can be prepared in such a way as to produce a mixture of crystalline and amorphous regions.crystallinity, the higher the value of the elastic shear modulus. The phase angle lies at 2° to 3°, so that the polymer is now close to a perfect elastic solid.
Glass Transition
The glass transition is reached during further cooling. Stiffness continues to increase but not as significantly as duringCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization (3.0E+07 Pa∙s at 200°C to 1.6E+08 Pa∙s at 140°C, figure 4).
While theGlass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery. glass transition temperature is usually evaluated by means of the peak temperature, which is typical for the curves of G" and δ (figure 5), cooling over the glass transition is also related with an increase in the G' curve. At temperatures lower than theGlass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery. glass transition temperature, the phase angle decreases again and is close to 0. The polymer is in a glassy, stiff state.
Conclusion
This application example shows how DSC and rotational rheology complement each other. Both methods provide different information describing theCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization and glass transition of semi-crystalline polymers, thus providing a comprehensive insight into the material behavior during heating and cooling. The typical detected effects are summarized in Tables 2a and 2b.
Table 2a: Typical effects measured duringCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization and glass transition of a semi-crystalline polymer by means of the DSC 300 Caliris®
1 in accordance with DIN ISO 11357-5:2014
2 in accordance with DIN ISO 11357-2:2014
Table 2b: Typical effects measured during crystallization and glass transition of a semi-crystalline polymer by means of the Kinexus rotational rheometer
| Measured curve | Complex shear viscosity | Elastic shear moduus G' | Viscous shear modulus G" | Phase angle δ |
|---|---|---|---|---|
Before the crystallization (melt state) | Temperature dependence of stiffness in the liquid state No effect | G' < G" The "liquid-like" properties dominate, the polymer flows | >45°: The lower the value, the more elastic the molten polymer is. | |
| CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization process | Strong increase (more than 3 times due to Tg). | Increase | Decrease from δ > 45° to δ < 45° | |
| CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization temperature | Midpoint | Crossover G'/G" | δ = 45° | |
| Between Tc and Tg; rubbery plateau | Temperature dependence of the stiffness in the rubbery plateau. No effect. | G' > G" The "solid-like" properties dominate, the crystalline phase gives a structure to the polymer, no flowing. | δ < 45° The lower δ, the stiffer the sample | |
| Glass transition | Increase | Increase | Peak: Glass transition temperature | Peak: Glass transition temperature |
| After Tg: Solid state | Temperature dependence of the stiffness in the solid state | - | - | Minimum value of δ |