
05.08.2020 by Dr. Gabriele Kaiser
Четкая идентификация сырья (RMID) с помощью ДСК
Идентификация сырья или контроль поступающих товаров имеет первостепенное значение для качества и безопасности лекарственных препаратов, где бы ни использовались химические вещества в фармацевтической промышленности - в качестве продукта химических реакций или основы для дальнейшей переработки.
Характерными величинами, которые можно использовать для идентификации вещества, являются
- Температура плавления/перехода
- Энтальпия плавления (теплота плавления)
- Энтальпия перехода
- Температура стеклования
- Удельная теплоемкость
Все они могут быть определены с помощью дифференциальной сканирующей калориметрии (ДСК). Температура и энтальпия плавления характерны для кристаллических веществ, включая полиморфы; температура стеклования - атрибут аморфных материалов. Полукристаллические вещества проявляют эффекты как плавления, так и стеклования.
Характеристика фенацетина
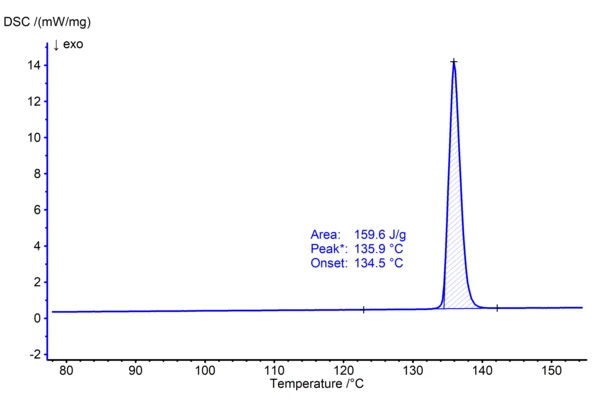
Фенацетин был представлен в 1887 году в качестве анальгетика, но из-за его вредного, особенно разрушающего почки действия в сочетании с другими обезболивающими препаратами, в большинстве стран этот препарат больше не продается в качестве лекарственного средства. Однако чистый фенацетин входит в набор чистоты для термического анализа от NIST (National Institutes of Standards and Technology, SRM 1514) и поэтому хорошо подходит для иллюстрации определения температуры плавления. В следующем эксперименте 1,04 мг фенацетина нагревали в атмосфере азота в алюминиевых тиглях при скорости нагревания 10 К/мин до 160°C. Кривая ДСК (рис. 1), полученная в результате этого измерения, показывает эндотермический эффект, который представляет собой плавление вещества. Глава USP <891> рекомендует соотносить экстраполированную температуру начала эффекта ДСК (в данном случае 134,5°C) с температурой плавления кристаллического материала. Для этого используется термин "температура начала плавления". Соответствующая энтальпия плавления (или теплота плавления) рассчитывается путем интегрирования пиков, в результате чего получается 159,6 Дж/г. Оценка может быть выполнена автоматически с помощью AutoEvaluation функциональные возможности программного обеспеченияNETZSCH Proteus® . Оба результата хорошо согласуются со значениями, приведенными в литературе (1) и (2).
Литература: (1) H. Manzo, A.A. Ahumada, J. Pharm. Sci., 1990, 79, 12, pp 1109 - 1115 (407,2 K) (2) A. Pen͂a, B. Excalera, A. Reillo, A.B. Sánchez, P. Bustamante, J. Pharm. Sci., 2009, 98, 3, pp 1129 - 1135 (28,75 кДж/моль, исходя из молярной массы 179,21 г/моль).