
31.05.2021 by Dr. Natalie Rudolph, Dr. Stefan Schmölzer
How Specific Heat Capacity of Filled Powders Affects SLS Processing Parameters
The modification of Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering (SLS) powders with fillers is a good way to modify the properties of the produced parts without the necessity for new powder materials. Learn how to assess the effect of copper fillers on the processing behavior.
Such filler systems are materials with higher electrical or Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity such as aluminum or copper. If a higher Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity is achieved, thermal management applications are in reach that can be further enhanced by the complex geometries possible with SLS. While the changed performance is desired in the final component, adding fillers to SLS powders also has an effect on the processing behavior and needs to be understood to successfully finish a build job.
Why copper is suitable
Take, for example, copper as a good heat conducting material. Its specific heat capacity is in the range of 0.4 J/g×K. Mixing it with PA12 powder must lead to the reduction of the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of the mixture. Therefore, the ability of the compound to store heat is reduced, heat is discharged faster and the thermal balance of a build can be changed. Learn more about cp measurements on unfilled PA12 powders here!
Preparing the samples for analysis
In a study at the Institute of Polymer Technology (LKT) at the University of Erlangen-Nuremberg, different mixtures of copper spheres and flakes in varying contents were produced and processed in an EOS Formiga P110 machine. The samples varied both in the form of the filler (spheres and flakes) and in the volume content (5 and 10 %).
The energy DensityThe mass density is defined as the ratio between mass and volume. density1 of 0.043 J/mm2 was kept constant for all materials to detect any changes in the process behavior due to the fillers. During processing, no samples could be produced with the 10 vol% copper flakes. The process temperature for the mixture with copper spheres was determined to be 167°C and with the copper flakes 173°C, respectively.
Measuring specific heat capacity
A NETZSCH DSC 204 F1 Phoenix® was used to measure the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity cp as a function of temperature of these different mixtures of PA12 powder with copper particles in comparison to the neat PA12 material. The measurements were performed in accordance with ASTM E1269 and ISO 11357-4.
After an initial cooling step to -25°C, the temperature was increased to 215°C at 10 K/min. Two different samples were measured and the average was calculated. The following table summarizes the measurement conditions.
Table 1: Measurement conditions
| Sample pan | Concavus® Al, pierced lid |
| Sample mass | 11.55 mg |
| Calibration reference | Sapphire |
| Reference pan | Concavus® Al, pierced lid |
| Atmosphere | N2 |
| Gas flow rate | 40 ml/min |
| Temperature range and heating rate | -25 … 215°C at 10 K/min |
Analyzing the measurement data with a smart software
The analysis in the NETZSCH Proteus® software is shown in Figure 1. It shows the „apparent“ Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of a PA12 sample with 5 vol% copper spheres, superimposed by the effects for melting and Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition.
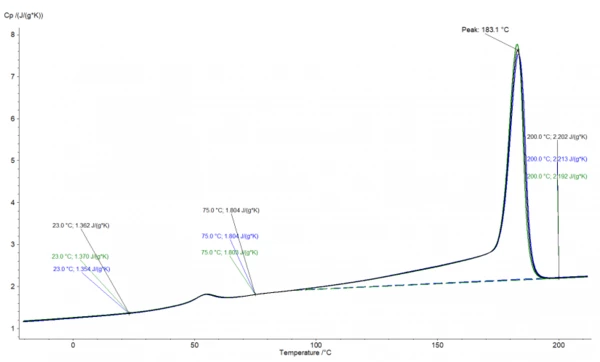
The cp data can be easily deduced from this curve. However, in the temperature range between 90-190°C, the effect of the increasing cp and the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect of melting are opposing each other. Therefore, the values in the melting range are typically interpolated.
Figure 2 shows the cp values after the interpolation for all four samples.
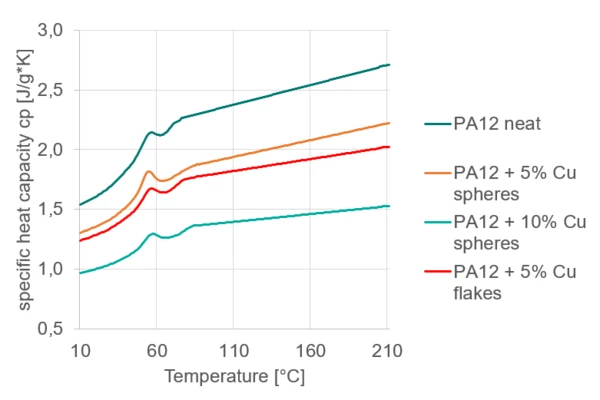
As expected, it can be seen that the cp is increasing with increasing temperature. The additional copper content reduces the cp and no effect of the filler geometry can be detected. The researchers at LKT even confirmed that the decrease in cp with increasing copper content follows the rule of mixture. However, they only measured the cp at 25°C. The temperature-dependent measurements shown in Figure 2 indicate further that the slope of the cp increase with temperature is slightly reduced the more copper particles are in the mixture.
The measurements confirm that the change in cp can contribute to the higher energy input required during 3D printing. However, additional information about the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity is needed to evaluate the impact of both effects on the thermal conditions.
It should be noted that this behavior is universal for all plastics materials modified with thermally conductive fillers. Therefore, it is an important quantity to be measured for the design as well as injection molding simulation of heat sinks or other components needed in thermal management.
About the Institute of Polymer Technology (LKT)
The Institute of Polymer Technology is an academic research institute at the Friedrich-Alexander University of Erlangen-Nuremberg. It is one of the leaders in Additive Manufacturing research; particularly SLS. Other main research areas include Lightweight Design and FRP, Materials and Processing, Joining Technology and Tribology. In addition to these research focuses, the institute is also working on cross-disciplinary topics such as Filler Material Compounding, Simulation of Processing and Applications, Radiation Cross-linked Thermoplastics, Gentle Processing and many more.
1Energy DensityThe mass density is defined as the ratio between mass and volume. Density = How much energy a system contains in comparison to its volume

FREE E-Book
Thermal Analysis and Rheology in Polymer Additive Manufacturing
Discover the secrets behind AM's game-changing capabilities! Our newly released ebook delves deep into the heart of AM, unveiling the power of reliable material characterization techniques, specifically thermal analysis and rheology.