Glossary
Specific Heat Capacity (cp)
What is specific heat capacity (cp)?
Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.
The equation as follows:
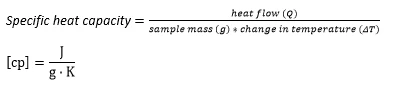
Specific heat capacity is the amount of heat needed to raise the temperature of one gram mass by 1 degree Celsius.
Specific heat capacity (cp) by DSC
By the equation
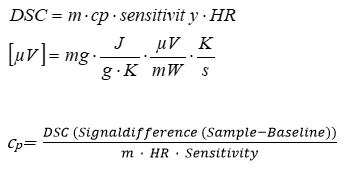
m = sample mass
cp= specific heat capacity
HR= heating rate
the specific heat capacity of a material can be calculated (according to e.g. DIN 51 007, ASTM E 1269 or ratio method) based on three measurements (baseline, sapphire, sample) by using DSC instruments (e.g. DSC 204 F1 , DSC 404 F1 ).
The sensitivity is derived from the calibration measurement using a sapphire as a specific heat standard.
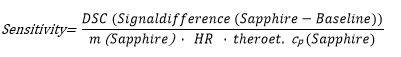
Specific heat capacity (cp) by LFA
The specific heat capacity is a thermophysical property with the SI unit of Joules per kilogram and Kelvin [J kg-1 K-1]. It defines a material's ability to store thermal energy.
Among other methods (e.g., differential scanning calorimetry, DSC), the specific heat capacity can be determined by using the laser flash technique (LFA). To this end, the LFA system is calibrated using a reference sample with known specific heat capacity. Sample and reference will be measured under exactly the same conditions (dimension, temperature program, graphite coating).
The Proteus® LFA analysis software calculates the temperature-dependent specific heat capacity along with the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity and Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of the unidentified solid material. LFA systems with a sample changer and a very fast plate or mini tube furnace (e.g., LFA 467, LFA 467HT) allow for simultaneous measurement of up to 16 samples over a broad temperature range within a few hours.

