Introduction
Magnesium stearate is a white powder used as a lubricant in the production of cosmetics and pharmaceuticals [5]. Its physical properties can vary from one batch to another because commercial magnesium stearate is a mix of different fatty acid salts that may vary in proportion [4]. Additionally, its properties heavily depend on its moisture content and hydration state [1]. The varying properties of magnesium stearate can be investigated by means of DSC, which is a particularly fast and easy method for obtaining a material’s fingerprint. Another thermal analysis method, TGA, can be used to indicate the hydration state of pure magnesium stearate. In the following, a magnesium stearate sample was characterized by means of DSC, TGA and PXRD (powder X-ray diffraction) measurements. In addition, the influence on the thermal properties of storage for two weeks in a humid atmosphere was studied.
Test Conditions
For the moisture treatment, the sample was stored in an open container placed above the water of a sealed water vessel for two weeks. The measurements were carried out with a DSC 214 Polyma and a TG 209 Libra® in a dynamic nitrogen atmosphere. Sealed Concavus® crucibles with pierced lid were used. The PXRD measurements were performed with the Bruker D8 Advance at solid-chem GmbH.
Test Results
The TGA measurements of magnesium stearate with and without moisture treatment are depicted in figures 2a and 2b (zoom of figure 2a).
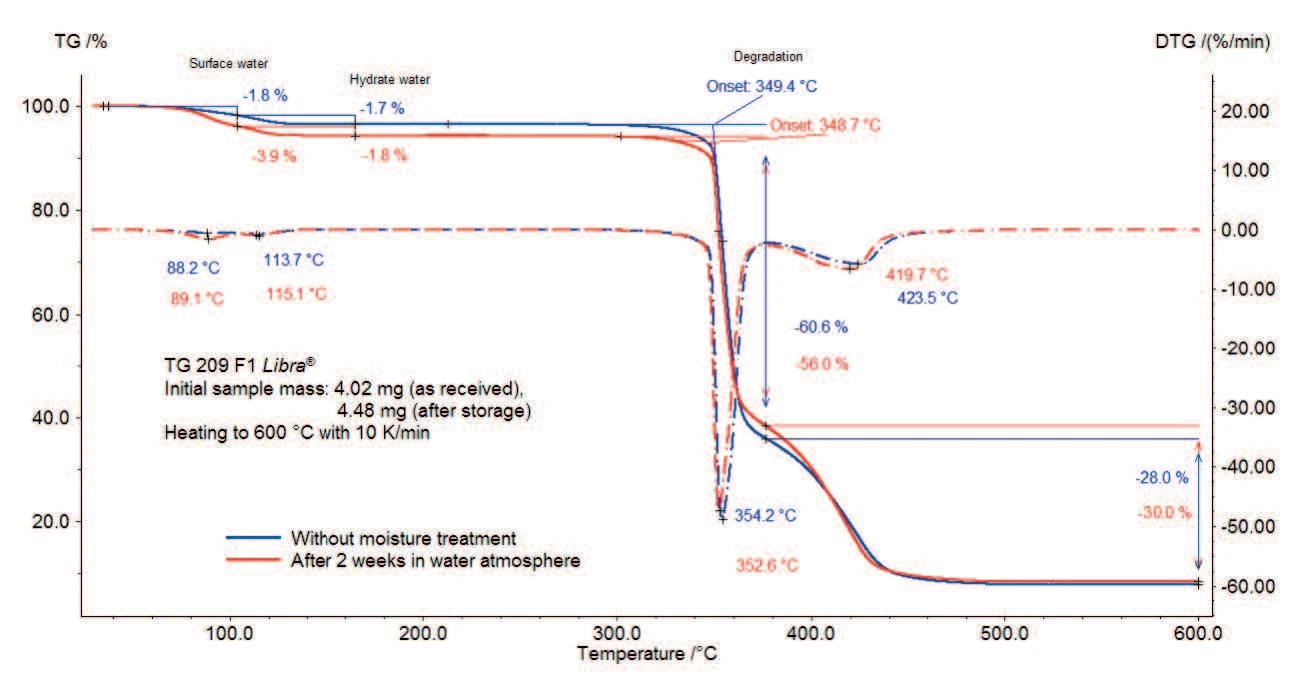
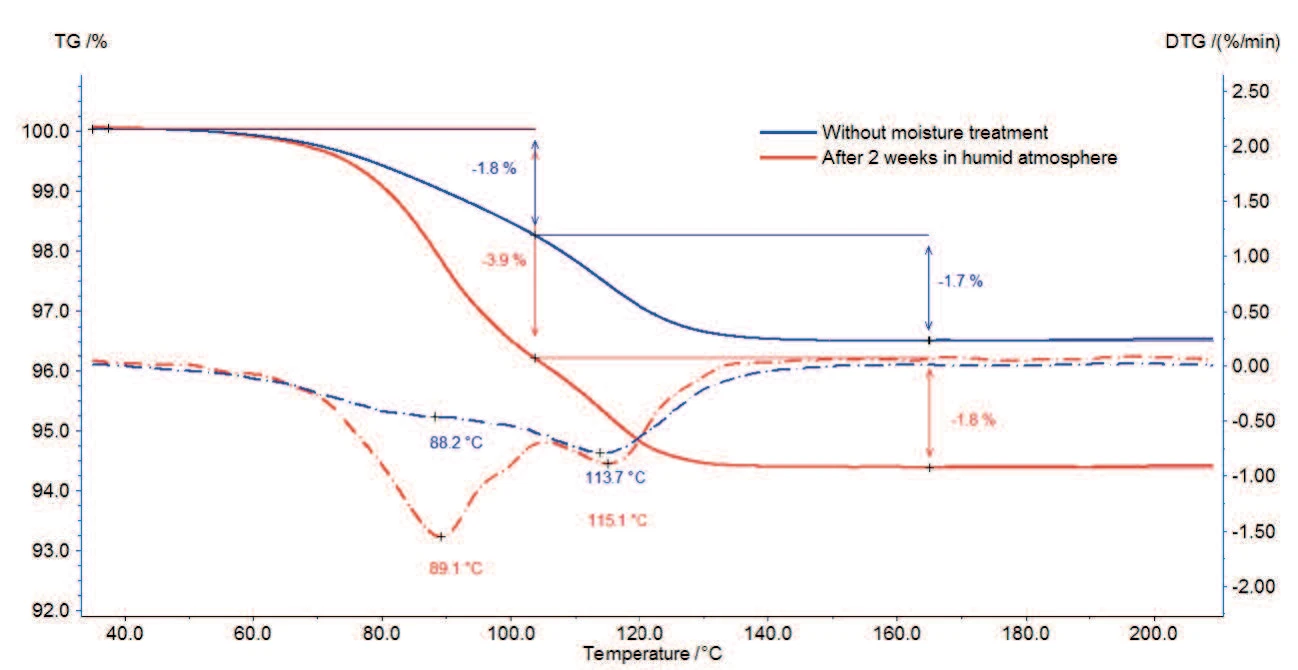
The sample loses 3.5% of its initial mass between room temperature and 130°C (continuous curve). It can be seen from the two peaks in the first derivative curve (DTG) in this temperature range that these processes run in two steps: the first mass-loss of 1.8% up to 100°C is due to the evaporation of surface water; the second mass-loss step amounts to 1.7% between 100°C and 130°C and corresponds to the release of hydrate water.
For the sample tested after storage in a humid atmosphere, both steps are also present but the first one is associated with a higher mass loss.
According to the results described in [6], the mass loss due to the release of the hydrate water begins around 65°C for the trihydrate, 85°C for the dihydrate and 95°C for the monohydrate form. Moreover, magnesium stearate has a molecular mass of 591.257 g/mol [2]. This results in a molecular mass of 609.257 g/mol for the monohydrate, 627.257 g/mol for the dihydrate and 645.257 g/mol for the trihydrate. Consequently, the loss of hydration water would be 2.95% for the pure monohydrate, 5.74% for the pure dihydrate and 8.37% for the trihydrate. This indicates that the sample without moisture treatment is a mixture of magnesium stearate at different states of hydration and additionally contains surface water.
Storage of the sample in a humid atmosphere leads to an increase in the first step resulting from the water release. According to [1], the moisture treatment has no influence on the hydration states of magnesium stearate. Consequently, after humidity treatment, the higher mass loss observed between room temperature and 130°C originates from adsorption of surface water or from water absorbed in the crystal structure.
The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of both samples starts around 350°C (extrapolated onset temperatures) and runs in two steps with a total mass loss of 89% (sample without storage) and 86% (sample after storage). The abruptness in the slope of the TGA curve between 350°C and 370°C indicates a fast reaction during the first Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition step.
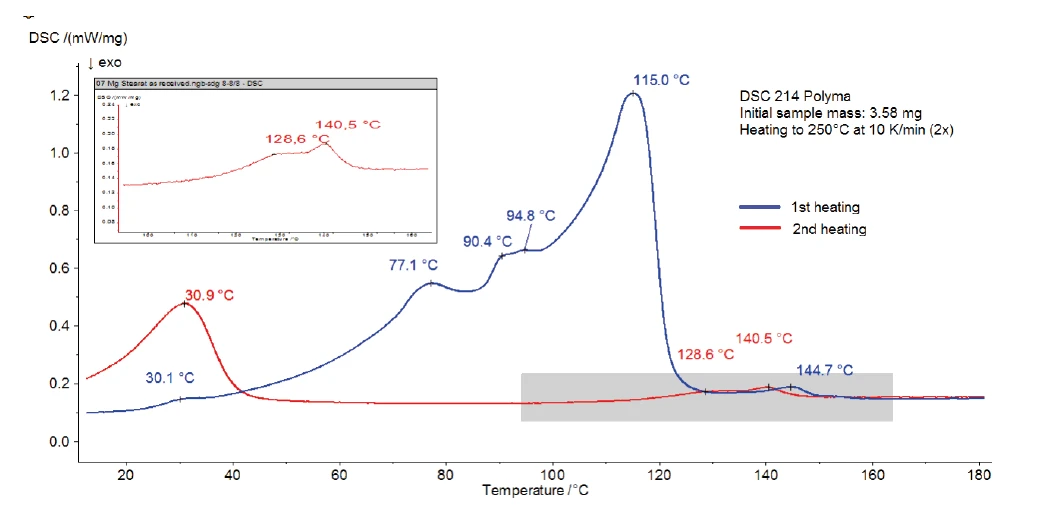
Figure 3 shows the DSC measurement of magnesium stearate without water treatment. A broad endothermal effect with peak temperatures at 77°C, 90°C and 115°C is detected between room temperature and 130°C. Part of it corresponds to the evaporation of water detected in the TGA curve. It may possibly be overlapped by melting of the sample, which also results in an endothermal peak. Some references indicate a melting range between 130°C and 145°C [3] and others a melting peak at 88°C [2]. Scattering of the data results from the fact that commercially available magnesium stearate very often consists of a mixture of different fatty acid salts than described above. With the variation of the individual components, the properties of the substance can vary from batch to batch [4].
The second heating (red curve) shows that only the peak at 31°C and the endothermal effect in the temperature range between 120°C and 150°C remain after the first heating. This indicates a reversible process, such as the melting of constituents. Due to the application of a pierced lid, the water (both adsorbed and chemically bound) is no longer present inside the sample at this temperature. Therefore, it is possible that the peaks at 145°C (1st heating) or 141°C (2nd heating) are related to the melting range of the water-free magnesium stearate.
Figure 4 depicts the DSC curves (1st and 2nd heating) of magnesium stearate after storage in a humid atmosphere. Compared to figure 3, the impact of the moisture treatment can easily be observed. It has a strong influence on the endothermal effects detected in the 1st heating between room temperature and 130°C, associated with differences in the physical properties as stated in literature [1].
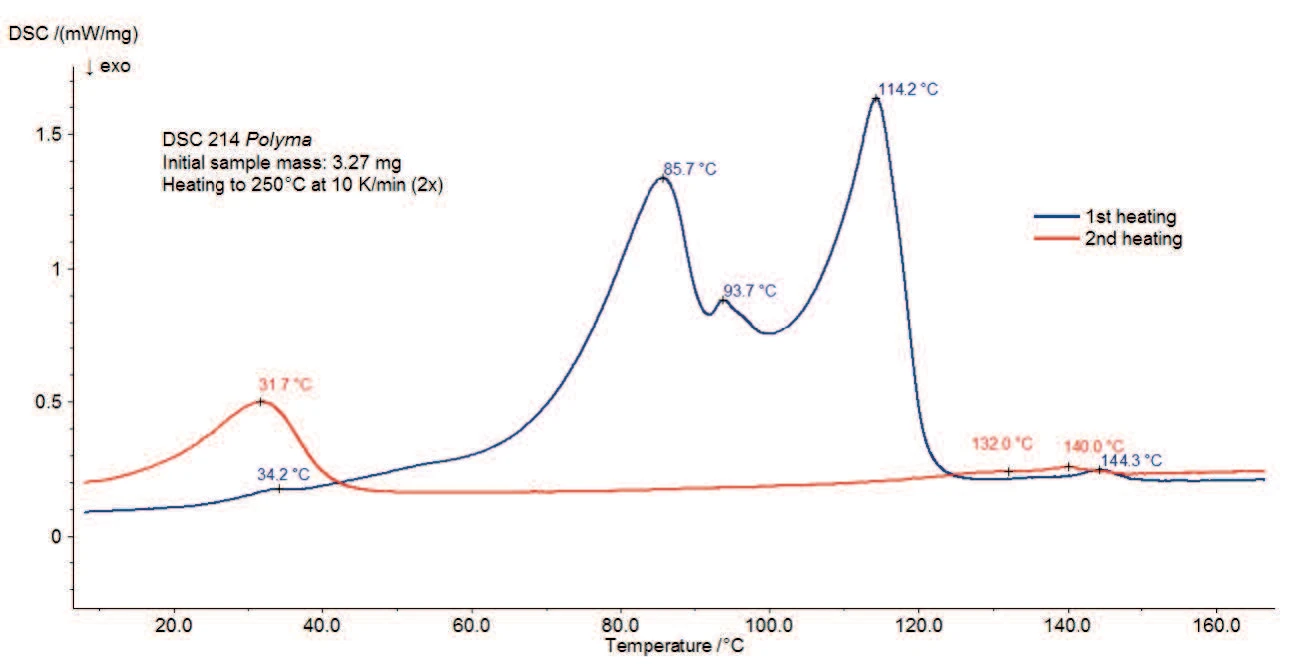
However, the second heating is very similar to that of the original sample. After heating to 250°C and controlled cooling in a dry atmosphere, both samples reach the same state. The peaks detected are due to melting of constituents.
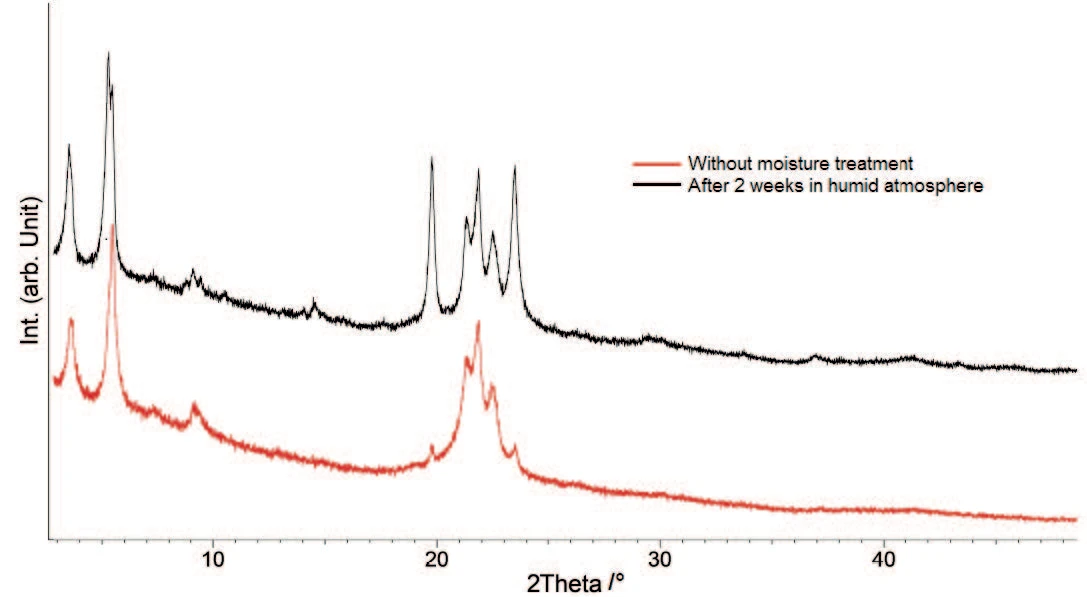
In order to be able to better characterize the individual components, X-ray diffraction (PXRD) measurements were carried out on both samples – original and watertreated (figure 5).
The PXRD patterns differ clearly for the peaks at approx. 20° and 23.5° 2θ. They are present in both samples but their intensities increase with moisture treatment. This means that a hydrate, which was already present in the original sample, is increasingly formed during storage in a humid atmosphere. The comparison of the X-ray pattern with literature data [6] confirms the trihydrate while concentrating on the peaks at 20° and 23.5° 2θ.
The hydrate forms are stable in presence of moisture [1], so that the trihydrate is formed from an anhydrate present in the original sample. This result confirms the assessment from LV Allen and PE Luner [7] that the anhydrous form of magnesium stearate rehydrates to form a trihydrate at relative humidity higher than 50%.
Conclusion
DSC and TGA measurements were carried out on magnesium stearate with and without storage in a humid atmosphere. The water treatment allowed for both the surface water and the crystal water to increase.
This knowledge is all the more important because there is a correlation between the moisture treatment and the physical properties of magnesium stearate [1]; therefore, it is crucial to conduct checks on the product before processing. To this end, DSC and TGA are useful tools allowing for fast characterization and/or comparison of the different lots.
Acknowledgment
NETZSCH would like to thank solid-chem GmbH in Bochum, Germany for carrying out the PXRD measurements and evaluation.