Introduction
Modulated DSC measurements are used to separate overlapping effects. The sample is subjected not only to linear heating rate, but also to sinusoidal temperature variations. This method leads to separation of the socalled reversing and non-reversing parts of the heat flow. The reversing effects are a function of temperature and oscillate with temperature variations. The non-reversing processes are a function of time and are calculated as the difference between the total heat flow and the reversing heat flow.
A modulated measurement contains three parameters to be chosen by the user:
- The underlying heating rate (in K/min)
- The amplitude (in K)
- The period of oscillation (in s)
An appropriate heating rate and a sufficient frequency are necessary to ensure that the effects to be separated contain enough oscillations for an improved separation of the effects. This is a required condition for achieving good separation of the reversing and non-reversing processes. Because it is difficult for a heat-flow DSC to follow fast heating rates along with short oscillations, modulated measurements are usually carried out at heating rates of less than or equal to 5 K/min.
Temperature Modulation with High Heating Rates
Thanks to the low thermal mass of the P-Module furnace, the heat-flow DSC 300 Caliris® can be modulated at heating rates of 10 K/min in combination with short periods and high amplitudes for results that are both quickly achieved and accurate.
In the following, a temperature-modulated DSC measurement is performed on a polystyrene sample. Table 1 summarizes the test conditions.
Table 1: Measurement conditions
| Device | DSC 300 Caliris® with P-Module |
|---|---|
| Crucible | Concavus® (aluminum, closed with pierced lid) |
| Sample mass | 5.25 mg |
| Temperature range | -20°C ro 150°C |
| Heating rate | 10 K/min |
| Period | 20 s |
| Amplitude | 1 K |
Measurement Results
The total measured heat flow (which conforms to a conventional DSC curve) is displayed in figure 1. The endothermic step detected at 84.5°C (midpoint) is due to the Temperatura de Transición VítreaThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polystyrene. It is overlapped with a RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation peak at 89.7°C resulting from the release of mechanical tensions within the sample. The two effects can only be evaluated if they are separated. This can be achieved using temperature modulation.
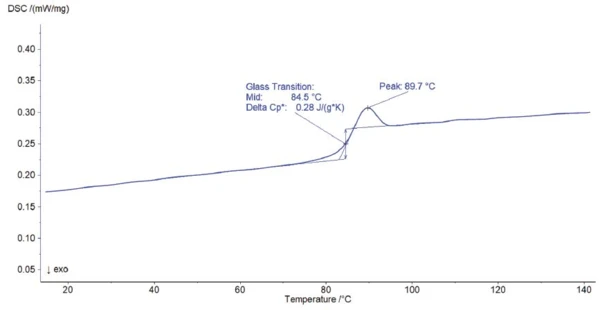
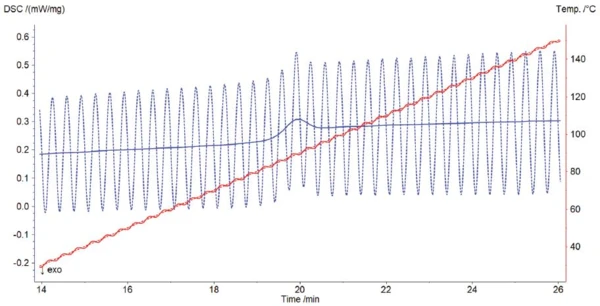
Figure 2 shows that the temperature is perfectly controlled during the modulated measurement: The underlying heating rate of 10 K/min and amplitude of 1 K are both maintained without any difficulty.
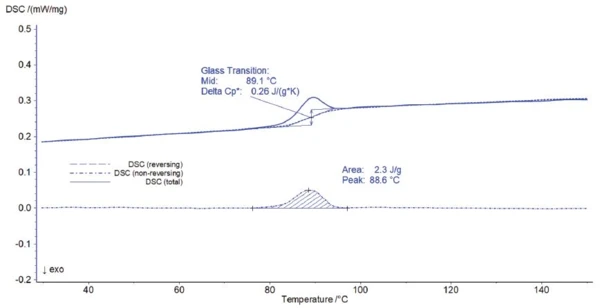
Separation of the total heat flow into reversing and non-reversing signals is shown in figure 3. The glass transition occurs in the reversing part of the heat flow whereas the irreversible RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation peak is a typical non-reversing effect. Both effects can now be correctly evaluated: The glass transition was detected at 89.1°C (midpoint) and the RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation peak at 88.6°C (peak temperature) with an enthalpy of 2.3 J/g.
Conclusion
Thanks to modulation at higher heating rates than usual, the glass transition of polystyrene can be quickly and accurately evaluated. The DSC 300 Caliris® with P-Module combines the robustness of a heat-flow DSC and the advantages of a fast, well-controlled furnace even allowing for temperature-modulated DSC measurements at high heating rates.