Introduction
Polymer blends are the combination of two or more polymers. They are combined to create a new material with different physical properties than their raw materials. They can be cost-effective alternatives to expensive technical polymers. Blends of ABS and PC are widely used as housings for electrical devices and appliances as well as in the automotive industry for interior panels. These blends combine excellent processing properties with high heat resistance and impact resistance superior to the individual components. For even higher toughness, blends of PA6 and ABS can be used. Another interesting example is the combination of POM and PTFE. The blend combines the properties of self-lubricating materials, a low coefficient of friction and improved wear-resistance properties by adding small amounts of PTFE to POM.
Therefore, these blends are used in tribological applications such as gear systems. While blends offer significant advantages during their service life, they make recycling at the end of life difficult. One of the most fundamental problems is the identification of the material as a blend as well as its composition to ensure it is sorted properly and can be reused if possible.
TGA-FT-IR Measurement and Interpretation
Identification of the components of a blend is often done by spectroscopic or chromatographic analysis. Also the combination of TGA and FT-IR can be a useful tool for identification of blends. On the one hand, the mass-loss steps give information about the polymer amount and PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis gases, detected by FT-IR, act as the fingerprint of the polymer and help with identification on the other.
Different blends were investigated with the PERSEUS® TG 209 F1 Libra® under the measurement conditions listed in table 1.
Table 1: Measurement conditions
| Sample | POM/PTFE | PA6/ABS | PC/ABS |
|---|---|---|---|
| Sample mass | 10.57 mg | 9.72 mg | 10.38 mg |
| Temperature program | RT - 850°C | RT - 850°C | RT - 850°C |
| Heating Rate | 10 K/min | 10 K/min | 10 K/min |
| Gas atmosphere | Nitrogen | Nitrogen | Nitrogen |
| Gas flow rate | 40 ml/min | 40 ml/min | 40 ml/min |
| Crucible | Al2O3 (85 μl), open | Al2O3 (85 μl), open | Al2O3 (85 μl), open |
Figure 1 depicts the obtained TGA-FT-IR data of the POM/PTFE blend. Two mass-loss steps of 92.6% and 1.3% were detected with peaks in the DTG curve at 366°C and 582°C. The Gram Schmidt signal, displaying the overall IR-changes, behaves like the mirror image of the DTG. Maxima were observed in the same temperature region.
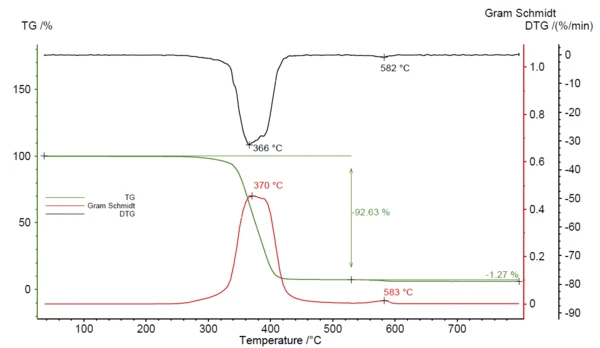
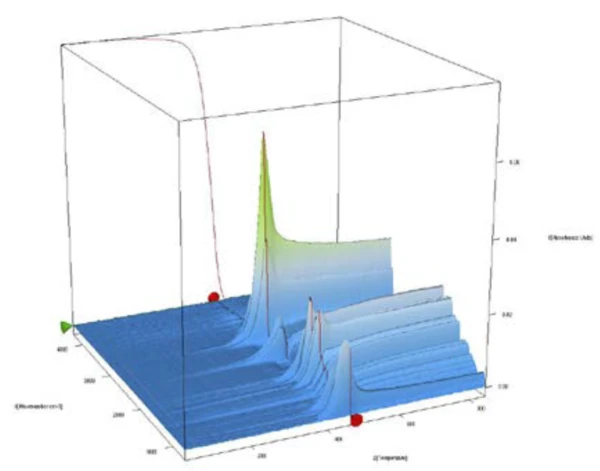
The complete IR data of the POM/PFTE blend is shown in figure 2 in a temperature- and wavenumber-dependent 3D plot. The TGA curve is plotted in red at the back and shows the correlation of the mass loss to the increase in IR intensity. For identification of the evolved gases, the single spectra are extracted and compared to the NETZSCH FT-IR database of polymers, which consists of PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis spectra of common polymers. The 2D spectrum during the first mass-loss step was in good accordance with the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis gases of POM (green).
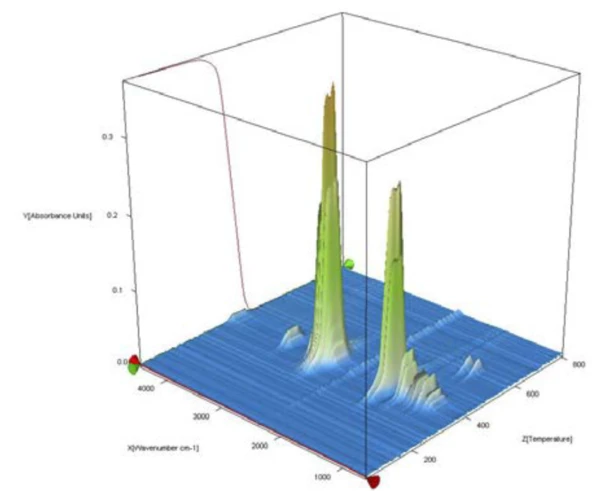
PTFE Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products (orange) were found during the second mass-loss step, compare figure 3. From this analysis, it can be concluded that the investigated blend was mainly made of POM with some minor amount of PTFE.
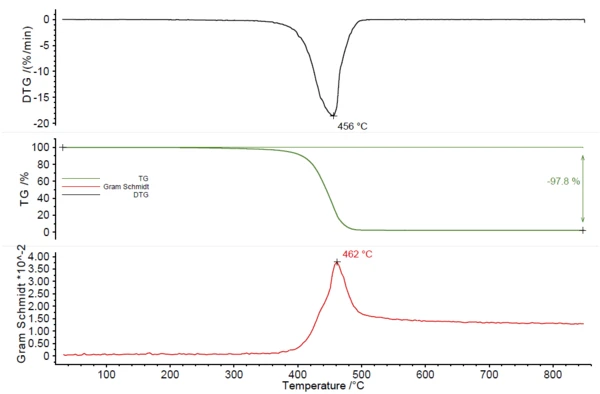
The second exemplary blend which was investigated, was a mixture of PA6 and ABS. Figure 4 displays the TGA curve with a mass loss of 98% and the Gram Schmidt curve with a peak at 462°C. From these curves, it could not be seen that the investigated sample consists of more than one material. Only evolved gas analysis can give more insight.
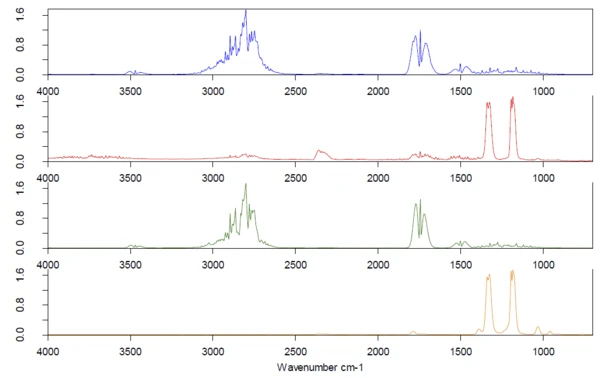
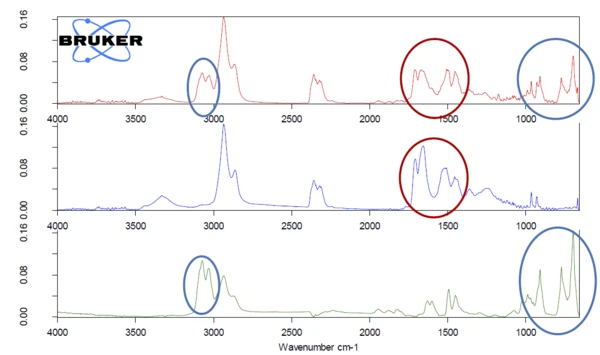
The 2D spectrum was extracted at 456°C (red) and compared to the NETZSCH FT-IR database of polymers, see figure 6. This comparison clearly reveals that the measured spectrum is a mixture of more than one polymer. PA6 was found with the highest similarity. After the spectrum subtraction, ABS was found as the second compound of this mixture. The red circles show unique VibrationA mechanic process of oscillation is called vibration. Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. In many cases, vibration is undesirable, wasting energy and creating unwanted sound. For example, the vibrational motions of engines, electric motors, or any mechanical device in operation are typically unwanted. Such vibrations could be caused by imbalances in the rotating parts, uneven friction, or the meshing of gear teeth. Careful designs usually minimize unwanted vibrations.vibration bands for PA6 in the measured spectrum, whereas the blue circles mark characteristic bands for ABS.
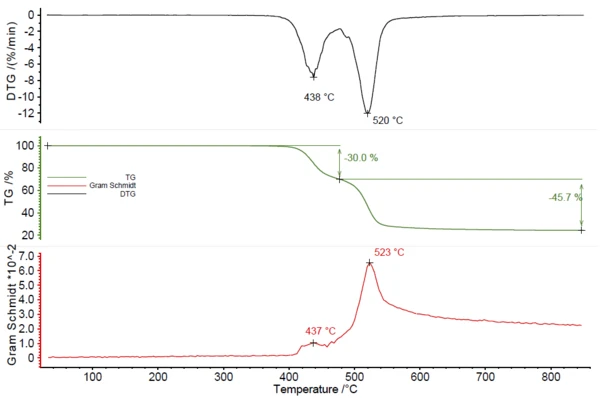
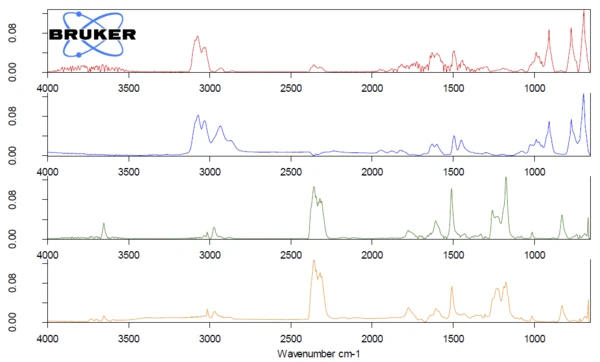
The third mixture of ABS and PC could also be easily identified by TGA-FTIR coupling. Figures 7 and 8 show the obtained measurement data. Two overlapping mass-loss steps of 30.0% and 45.7% were detected with peaks in the DTG curve at 438°C and 520°C. The Gram Schmidt curve shows peaks at the same temperatures. The comparison of the measured spectra at these temperatures with the NETZSCH FT-IR database of polymers gave good accordance with ABS for the first mass-loss step and PC for the second mass-loss step.
Conclusion
These examples show that the hyphenation of TGA and FT-IR is a very suitable tool to identify polymer blends. TGA curves enable quantification of the polymer content, whereas identification of the polymers is done by means of the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis gases compared to the gas phase library NETZSCH FT-IR Database for Polymers. It is a good solution when quantifiable results are needed. Especially when the polymer is black, which can make FT-IR analysis via ATR difficult. Limitations may occur when PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis gases interact and form new molecules, which differ from the compounds released from pure polymers.