POLYMERS
Curing of EVA
The most widely used encapsulant is EVA (ethylene vinyl acetate copolymer), due not only to its high Electrical ResistivityElectrical resistivity or electrical resistance is a fundamental material property that quantifies how strongly a given material opposes the flow of electric current.electrical resistivity, low fusion and polymerization temperature, and low water absorption ratio, but also to appropriate optical transmission properties.
As the polymerization reaction is irreversible, the thermal treatment of the PV cell encapsulation is crucial.
The quality and lifetime of the PV modules/arrays depend on the caliber of this production process.
In this example, Dielectric Analysis of an EVA sample was carried out in the lab furnace of the DEA. The DEA system is optimally designed for materials with standard to long Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing times (> 3 min).
Time and temperature ramps can be easily programmed at heating rates of up to 40 K/min. In addition, all disposable comb sensors can be used in the furnace to ensure a broad application range of the system setup.
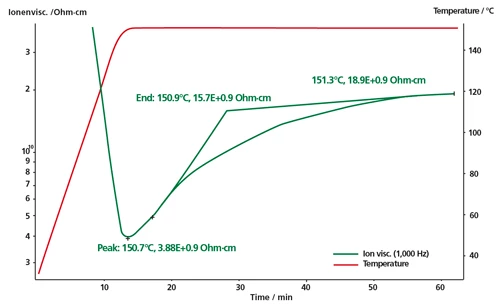
The multi-frequency measurement (with frequencies between 1 Hz and 10000 Hz) was carried out, and the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.ion viscosity (Ω.cm) was monitored. Presented here is the behavior of the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.ion viscosity at 1 Hz.
The cross-linking reaction by using peroxide was observed under IsothermalTests at controlled and constant temperature are called isothermal.isothermal conditions at 150°C.
The increase in the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.ion viscosity correlates with the increase in the Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). degree of cure. After 60 min, the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.ion viscosity remains nearly constant, which indicates that the cross-linking reaction has essentially finished.