STA/TG-DSC
Simultaneous Thermogravimetry – Differential Scanning Calorimetry
Simultaneous Thermal Analysis (STA) is based on standards, e.g., ISO 11358, ASTM E793, DIN 51004, DIN 51006, DIN 51007. It generally refers to the simultaneous application of Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC) to one and the same sample in a single instrument.
The simultaneous application of Thermogravimetry (TG) and Differential Scanning Calorimetry (DSC) to a single sample in an STA instrument yields more information than separate application in two different instruments:
- The test conditions are perfectly identical for the TG and DSC signals (same atmosphere, flow rate, vapor pressure on the sample, heating rate, thermal contact to the sample crucible and sensor, radiation effect, etc.).
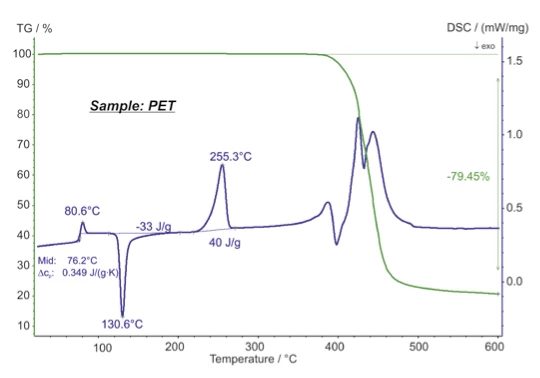
- The analyzability of the signals is improved, since two or more sets of information concerning sample behavior are always simultaneously available (differentiation between phase transformation and Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition, between addition and condensation reactions, recognition of PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis, OxidationOxidation can describe different processes in the context of thermal analysis.oxidation, and combustion reactions, etc.)
Since the early years of its existence, NETZSCH-Gerätebau GmbH has given high priority to the development and continuing optimization of its Simultaneous Thermal Analyzers; these have today taken on a market-leading position as a result of their perfect technology.
The respective instrument and application standards for TGA and DSC (see respective sections) are of course also met by NETZSCH STA instruments.
The New STA 509 Jupiter® Series
The modular design of the STA allows for easy exchange of furnaces and sensors to accommodate multiple applications over a wide temperature range from -150°C to 2400°C. The top-loading design provides ideal performance and easy of use, making it the obvious choice for a flexible analytical system and evolved gas analysis. The vacuum-tight design and meticulous control of gas flows allow precise handling of high-purity atmospheres with respect to various inert, oxidizing, reducing and corrosive gases.
A wide range of accessories, including humidity and water vapor generators, expand the application possibilities. Furthermore, the integration of Evolved Gas Analysis with MS, FT-IR, or GC-MS systems significantly enhances the analytical potential of the STA 509 Jupiter® series.
- STA 509 Jupiter®Classic

Best Price/Performance Ratio
- RT to1600°C
- SiC furnace
- Balance resolution: 0.1 μg
- Optional 20-position ASC
- STA 509 Jupiter®Select

Taylored to Your Needs
- -150 to 2400°C
- Choice of 12 different furnaces
- Balance resolution: 0.1 μg
- Optional 20-position ASC or 2nd furnace
- STA 509 Jupiter®Supreme

Instrument for Highest Performance
- -150°C to 2000°C
- Choice of 9 different furnaces
- Balance resolution: 0.025 μg
- Optional 20-position ASC or 2nd furnace
- H₂Secure

Safe Examination of Materials Under Hydrogen
- Accessory for STA 509 Jupiter® series
- Retrofittable for STA 449 Jupiter® series
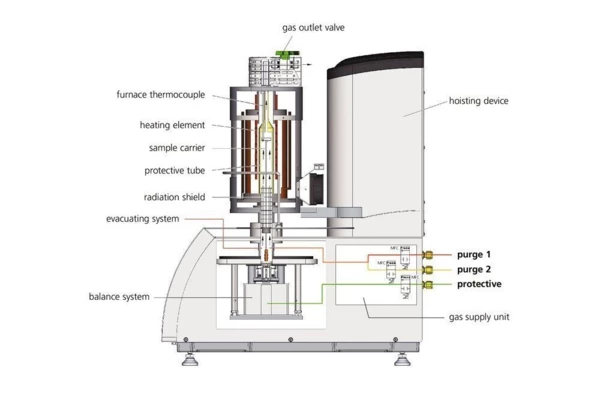
The advantages are obvious:
The test conditions are perfectly identical for the TGA and DSC signals (same atmosphere, gas flow rate, vapor pressure on the sample, heating rate, thermal contact to the sample crucible and sensor, radiation effect, etc.). Furthermore, sample throughput Is improved as more information can be gathered from each test run.
More Details about this method
Application Literature

MEASUREMENT WANTED?
Our NETZSCH applications laboratory is providing contract testing services for a wide range of industries and research centers. It is equipped with state-of-the-art testing instruments allowing for a variety of thermal analysis measurements to be carried out.
Consult with the experts in our applications labs to choose the best-suited measuring method for your specific needs.