Introduction
Evolved gas analysis (EGA) coupled to thermal analyzers such as thermogravimetry (TGA) or simultaneous thermal analysis (STA) which refers to simultaneous TGA-DSC is well established since it greatly enhances the value of TGA or TGA-DSC results. The sensitive and selective Fourier Transfrom Infrafed (FT-IR) technique is in particular useful for the analysis of organic molecules but also for infrared-active permanent gases evolved during most Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition processes. Such permanent gases like CO2 or SO2 are gaseous at ambient conditions.
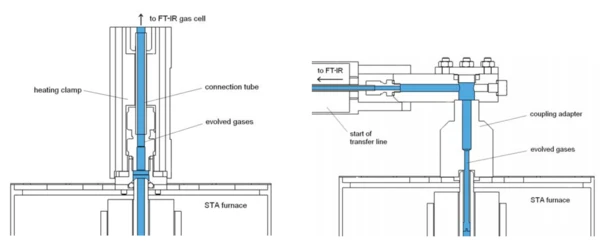
The coupling interface between thermal analyzers and FT-IR spectrometers is usually realized using heated adapters and a flexible, heated transfer line where the heating is required to avoid condensation of evolved gases on the way to the FT-IR instrument. Although integrated software solutions are available, thermal and gas analyzers are still physically separated. The path through the transfer line causes furthermore a delay between the release and the detection of the evolved gases and in some cases condensation or interaction eff ects.
For this work, the new direct Perseus coupling of an STA instrument and an FT-IR spectrometer without a transfer line was used [1]. A very small FT-IR spectrometer is directly mounted on top of the STA furnace leading to a compact and fully integrated STA-FT-IR coupling system called Perseus STA 449 (see figure 1). Perseus is a new member of the NETZSCH family of coupling systems as illustrated in figure 2.

The short interface to the STA furnace (see figure 3) as well as the gas cell of the FT-IR spectrometer is heated in order to minimize the risk of condensation. Furthermore, no liquid nitrogen is required since the FT-IR detector of DLaTGS type is operating at room temperature.
The basic instrument NETZSCH STA 449 F1 /F3 Jupiter® enables to measure high-resolution TGA and DSC or DTA simultaneously in a wide temperature range of -150°C to 2400°C depending on the furnace and sample carrier used.
Experimental
A PTFE/graphite compound with an initial sample mass of 11.54 mg was measured in Pt crucibles with pierced lids at a heating rate of 10 K/min. The gas atmosphere (flow rate 70 ml/min) was switched from pure argon to synthetic air at 870°C. A TGA-DSC sample carrier type S and a rhodium furnace were applied. The TGA-DSC results were baselinecorrected (empty-run signals were subtracted) and FT-IR acquisition was carried out at a resolution of 4 cm-1 and 16 scans were averaged for one FT-IR spectrum where one scan took about 1s.
Results and Discussion
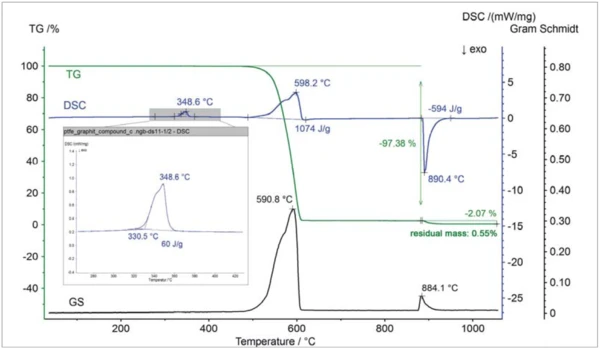
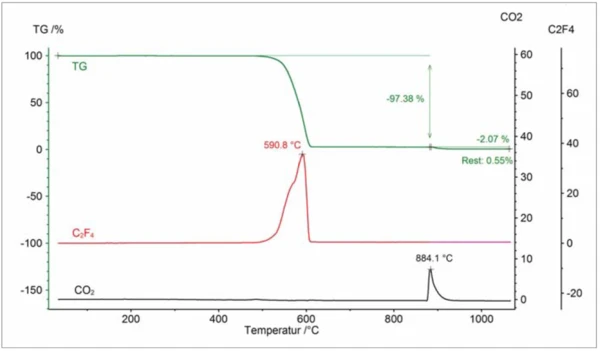
The Perseus coupling is well suited for many applications [1]. As an example, the results on the above mentioned PTFE/graphite compound – which can, for example, be applied as a lubricant – are shown [2]: figure 4 depicts the TGA-DSC results together with the Gram-Schmidt curve. The Gram-Schmidt curve portrays the intensity change of the entire IR absorption detected. At approx. 349°C (peak temperature), the DSC signal reveals an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect which is due to melting of the PTFE content. Between about 480°C and 620°C, a mass-loss step of 97.4% occurs together with an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic DSC effect and a peak in the Gram-Schmidt signal. In this range, pyrolytic Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the PTFE content takes place. At 870°C, the gas atmosphere was switched from inert to oxidative, leading to ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic burn-up of the graphite content of approx. 2.1%. The residual mass of about 0.6% is most probably due to a ceramic filler.
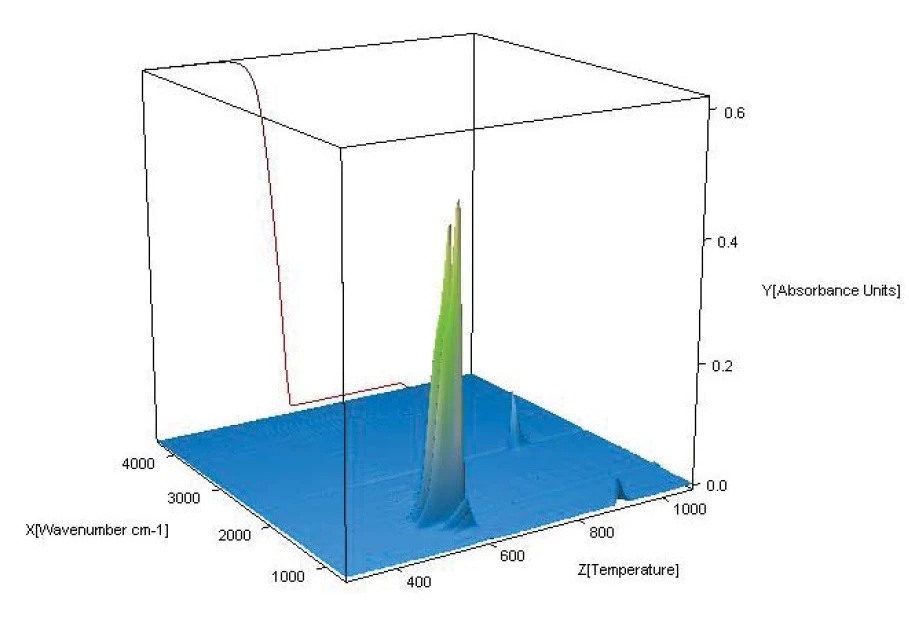
The “3-D cube” presented in figure 5 shows the IR absorption as a function of wave number and temperature, together with the TGA curve. During the first mass-loss step, the well-known absorption bands of tetrafluoroethylene, C2F4, can primarily be identified in the range between 1100 cm-1 and 1400 cm-1 (as well as traces of HF in the range between 4000 cm-1 and 4200 cm-1). The bands detected during the second mass-loss step, primarily in the range between 2200 cm-1 and 2400 cm-1, can be attributed to CO2 formed during combustion. Finally, figure 6 depicts the characteristic integration traces for C2F4 and CO2 as a function of temperature showing again an excellent correlation between mass-loss steps and evolved gases.
Conclusion
The application example presented demonstrates that Perseus allows for simultaneous recording of TGA and DSC and, at the same time, detection of the gases evolved by means of FT-IR. The entire STA-FT-IR results allow for quantification and identification of each sample component since initially unidentified gases can often be identified by means of a database search [1]. A very good correlation between the detected mass-loss steps and the gases evolved was shown which is a benefit of the direct coupling interface. All in all, the new Perseus STA 449 F1 /F3 is a high-performance, direct STA-FT-IR coupling without any transfer line that sets itself apart particularly by virtue of its compactness.