
05.04.2023 by Dr. Gabriele Kaiser
How Much Vanillin Is Contained in Vanilla Sugar?
A HAPPY EASTER BLOG POST
With spring comes the Easter season ‒ an opportunity for many people to take their favorite baking recipes out of the drawer and get creative in the kitchen. In many cases, the sweet floral flavor of vanilla lends a very special touch to cakes, biscuits/cookies, and other treats. It is no wonder that vanilla is one of the most frequently used flavors ‒ hardly any ice cream, pastry or dessert can do without vanilla.
For baking, this flavoring is mostly employed in the form of vanilla sugar or vanillin sugar. In the following, read how the two flavored sugar types differ and how the amount of vanillin in a commercial vanilla sugar package can be determined by means of differential scanning calorimetry (DSC).
Vanilla is a very complex flavoring agent. So far, more than 200 different flavor-active substances have been found in it. The main flavor or fragrance component is vanillin, chemically known as 4-hydroxy-3-methoxybenzaldehyde.
Did you know? Besides food, vanillin is also a popular additive for cosmetics and drugs. In total, it flavors about 18,000 different consumer goods [1].
The annual global demand for vanillin is estimated to be 15,000 tons. However, only about 35 tons can be extracted from vanilla beans (actually vanilla capsules) [1]. The vanilla plant is very challenging to grow, and the vanillin content of the capsules is usually less than 2.5% [2]. Therefore, most of the vanillin available is synthetically or microbiologically produced [1].

Vanilla Sugar or Vanillin Sugar?
While vanilla sugar is made of sucrose (household sugar) and crushed vanilla beans and/or vanilla extract, vanillin sugar is a mixture of sucrose and vanillin [3]. The latter is considerably more favorably priced and can be found as a standard baking ingredient in almost every supermarket.
In accordance with the BLL guideline [3], vanillin sugar in Germany must contain at least 0.1 g vanillin per 8 g total content immediately after production, i.e., 1.25%. This content may reduce to 0.085 g (= 1.06%) by the expiry (or “best-before”) date.
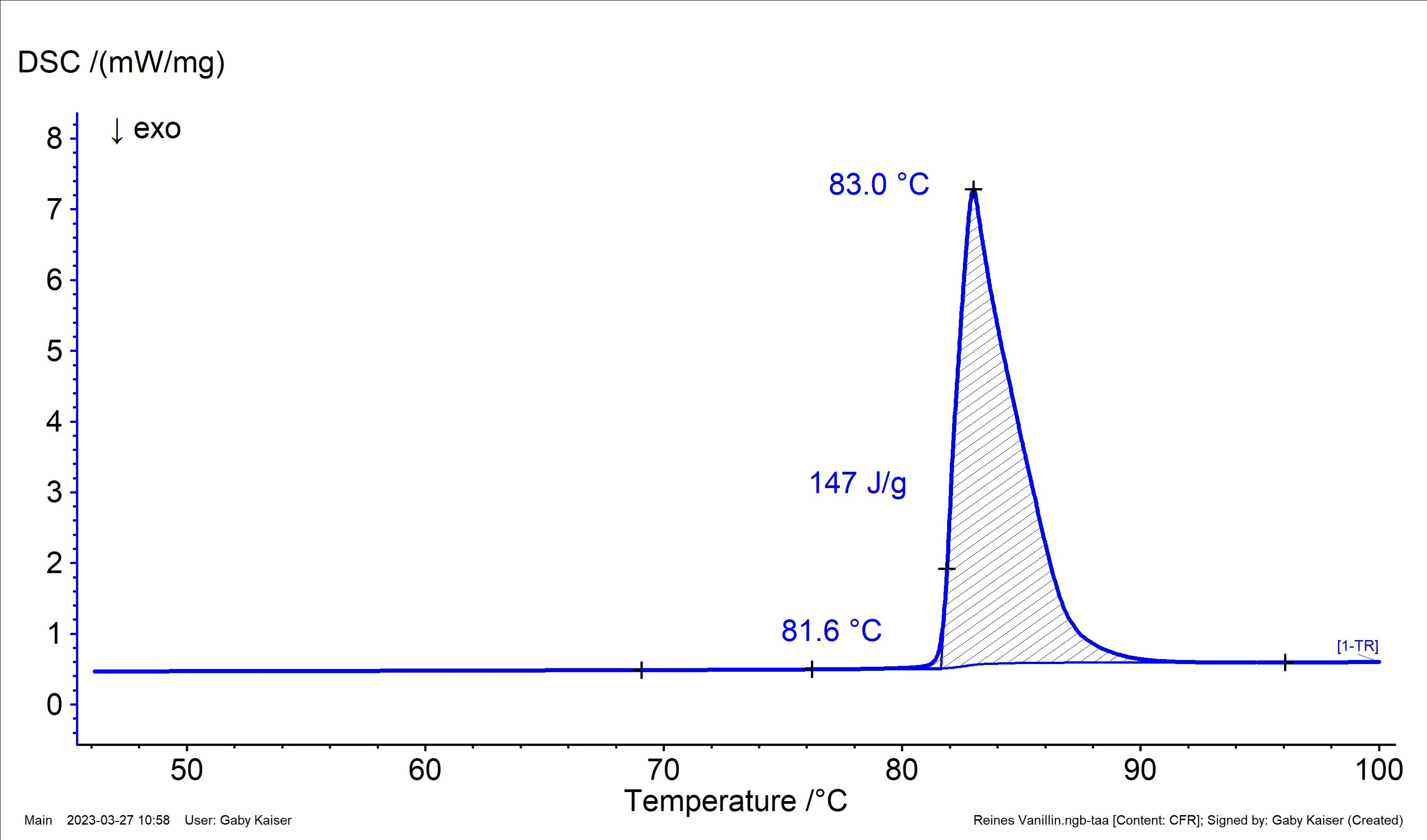
Pure vanillin is a white powder that can occur in two different crystal forms: Form I and Form II. Form I is the more thermodynamically stable one and melts at 82°C with a melting enthalpy (heat of fusion) of 22.4 kJ/mol or 147 J/g [4, 5]. As such, vanillin is also available as a certified Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature reference material.
Figure 1 shows the result of a DSC measurement on pure vanillin. Both the extrapolated onset temperature and the calculated heat of fusion are in good agreement with the theoretical values.
Vanillin in Vanillin Sugar
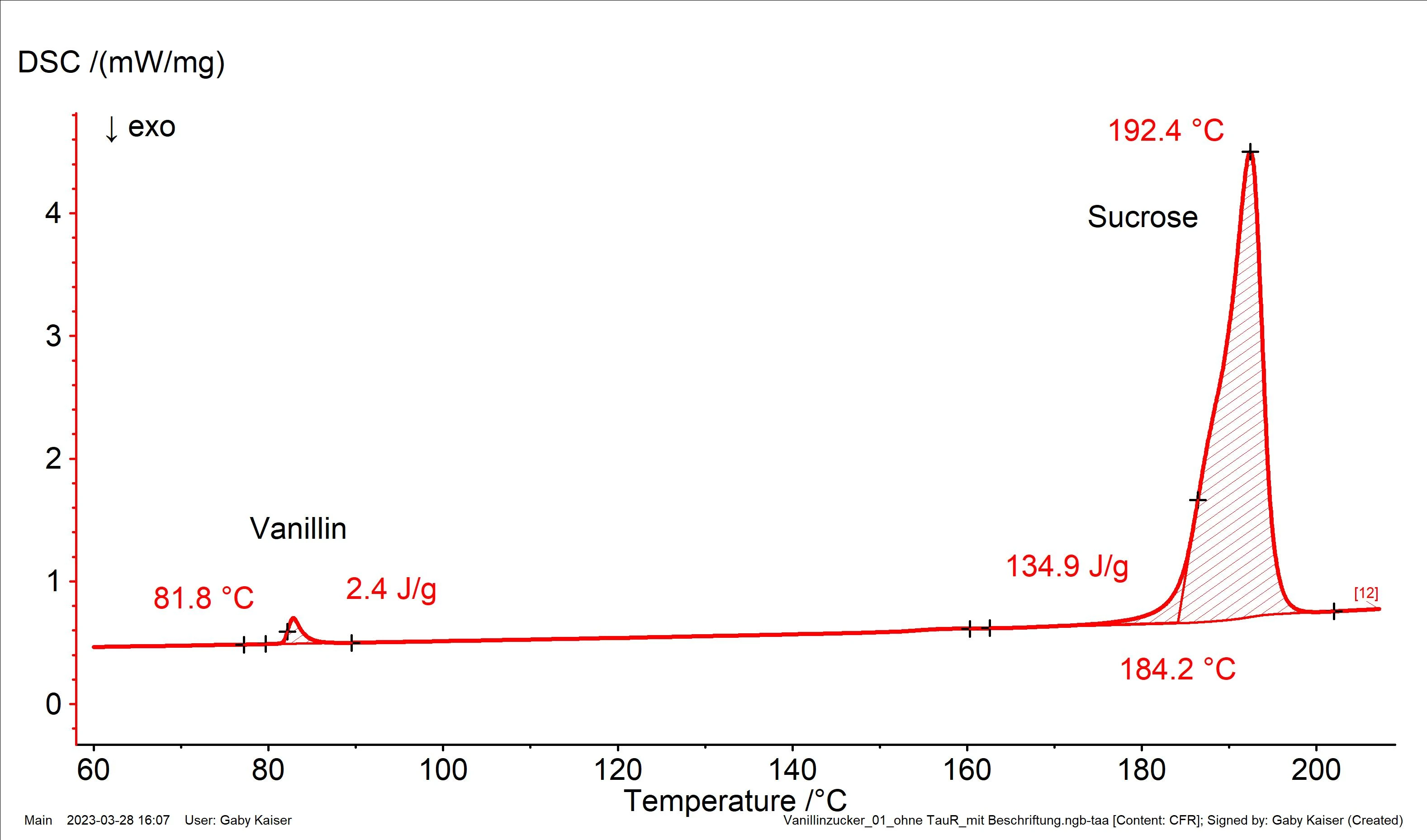
Figure 2 depicts the result of a DSC measurement on a commercially available vanillin sugar sample. Due to the very different melting ranges of vanillin (extrapolated onset temperature: 81.8°C) and sugar (extrapolated onset temperature: 184.2°C), the two components can be easily distinguished from each other.
Through comparison of the heat of fusion for vanillin determined in this experiment with the enthalpy of fusion for the pure substance, a vanillin content of about 1.6% is calculated.
In order to obtain a representative result, 10 samples were taken in total from a sachet of vanillin sugar and measured with a sample amount of between 5.1 mg and 6.1 mg (not shown here). The average proportion of vanillin was at approx. 1.86%, i.e., even slightly higher than the required minimum content.
Conclusion
If the characteristic effects of the components of a mixture are far enough apart and do not enter into any connection with each other, so that the associated temperatures and peak areas can be calculated independently, DSC can be used to estimate the respective contents by comparison with the data for the pure materials.
Despite the low proportion of vanillin in vanillin sugar (< 2%), the amount is enough to give the cake the desired flavor.
We hope you enjoy baking and wish you Happy Easter!
Literature:
[1] Aromawissen kompakt – der Aromastoff Vanillin, Deutscher Verband der Aromenindustrie e.V.
[2] Woher kommt Vanillin und wie stellt man es her? Schrift des Deutschen Verbandes der Aromenindustrie e.V.
[3] Richtlinie für Vanille-Zucker und Vanillin-Zucker (2007), Bund für Lebensmittelrecht und Lebensmittelkunde e.V.
[4] Wikipedia
[5] NIST chemistry webbook,
https:// webbook.nist.gov/cgi/cbook.cgi?ID=C121335&Units=SI&Mask=4#Thermo- Phase





