Introduction
Differential scanning calorimetry (DSC) allows for the determination of Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition temperatures and transition enthalpies, also for Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing reactions. The samples are usually analyzed in a crucible with pierced lid under normal pressure with a constant purge gas flow. Differential scanning calorimetry can also be used for investigation of photocuring reactions [1]. The combination of an UV lamp with the NETZSCH DSC 204 F1 Phoenix® (figure 1) features a versatile tool here.

Results
Single-Curing Systems: Comparison “Good/Poor“ of 2 Printing Inks
The sample is prepared in an open crucible which is irradiated by UV light. Intensity and irradiation time can be varied at a defined temperature program. IsothermalTests at controlled and constant temperature are called isothermal.Isothermal conditions or a dynamic temperature program are generally employed.
Figure 2 shows the results of the Photo-DSC for the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of an acrylate-based screen printing ink. Two samples of different lots were investigated. The experiment was carried out at a constant temperature of 35°C under a nitrogen atmosphere. Irradiation took place in a pulsed manner with UV pulses with an intensity of 1 W/cm² and a pulse time of 1 s. From the measurement, the conversion curve is calculated, assuming that during the last irradiation step, Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing no longer occurs. The last irradiation step was subtracted from the previous steps and the enthalpy of a single step was set proportional to the total enthalpy.
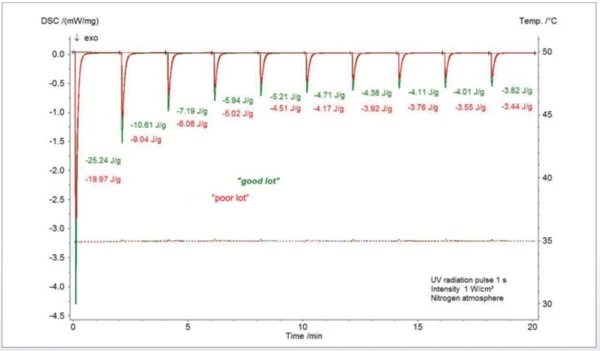
The conversion curve in figure 3 shows that there is a slight difference in the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing behavior of the “good” sample compared to the “poor” sample during the fi rst two irradiation steps.
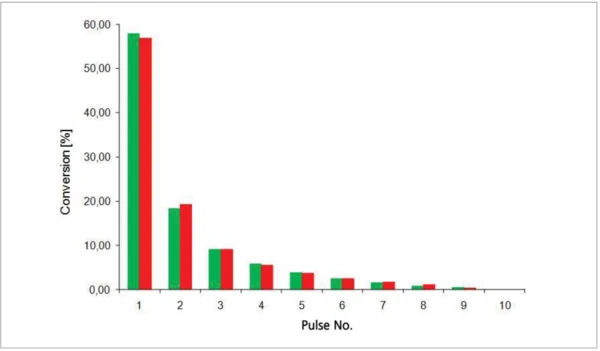
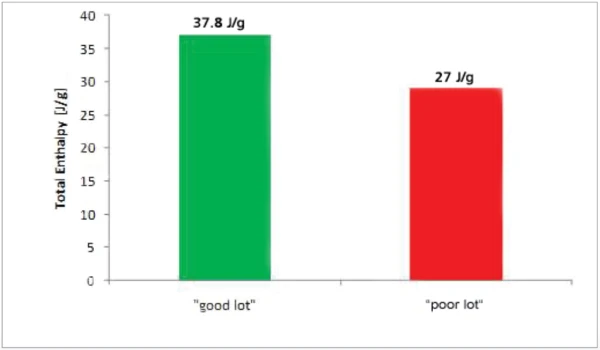
Figure 4 depicts the total enthalpies for the two inks which show significant differences. The “good” sample shows a higher reactivity compared to the “poor” sample.
Impact of the Gas Atmosphere
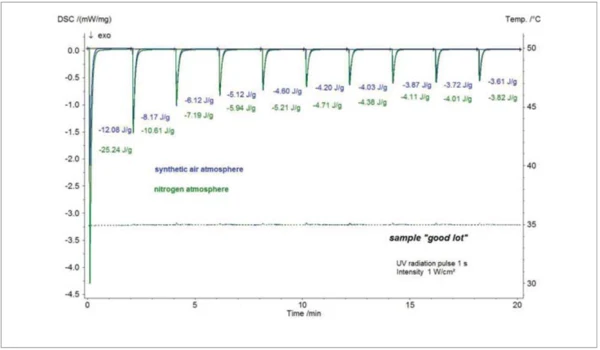
The influence of oxygen on the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing behavior is well known for acrylate systems. This is shown for the “good” screen printing ink in figure 5. UV-DSC measurements with different atmospheres could easily be realized with the NETZSCH DSC 204 F1 Phoenix® using the internal mass flow controllers for a precise purge gas flow. The results show that the enthalpy for Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing is lower compared to the measurement under a nitrogen atmosphere. The present oxygen acts as an inhibition agent for the UV-Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing process [2].
Influence of the Color on the Curing Behavior
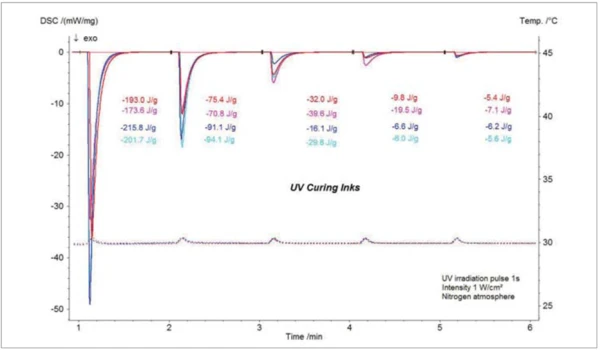
The blue curves in figure 6 represent the UV-DSC results for two blue inks and the red curves are the UV-DSC results for the red inks. Both blue inks (different lots) show a significantly higher enthalpy for UV curing compared to the red inks. Again, slight differences in the curing behavior of the two ink lots of the same color are monitored by the UVDSC results. Especially for the development of new formulations, UV-DSC results are a helpful tool to achieve formulations of different colors but with the same curing behavior which is necessary for the later application.
Results for a Dual-Curing System
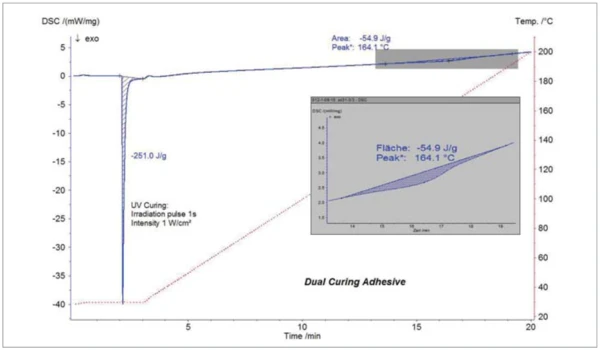
Along with the investigation on systems with a single curing mechanism, the UV DSC can also be used for dual-curing systems [3] like special types of adhesives. These kinds of adhesives do not only cure by UV radiation, they also show a thermal post-curing effect. Figure 7 shows the results for such a system. Radiation with UV light for 1 s at ambient temperature shows an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic curing effect with an enthalpy of 251 J/g. By heating the sample to 200°C, the thermal curing effect could be observed at 164°C (peak temperature) with an enthalpy of 55 J/g. This example clearly demonstrates that complete characterization of the curing behavior can be derived from one single UV-DSC experiment.
Summary
Differential Scanning Calorimetry (DSC) in combination with radiation of an UV lamp allows for the investigation of curing processes of UV-curing systems. The results obtained help gain an insight into curing mechanisms and the kinetics of curing reactions. In addition, dual-curing systems were investigated within one single experiment.