Introduction
Since the worldwide spread of the SARS-CoV-2 corona virus, mouth-nose protection has become part of our daily lives. Initially, bandanas, scarves and cloth masks were used in everyday life, but due to the rapid spread of the virus, they were replaced by medical masks such as surgical or FFP2 masks. Once mouth-nose protection is put on, it is constantly in the wearer’s inhaling and exhaling respiratory flows. The exhaling respiratory flow, in particular, is nearly saturated, with a humidity of 98% during exhalation [1]. As a result, the mask material is continually moistened, thus reducing the filter function. Furthermore, the humid environment promotes proliferation of harmful bacteria and fungi within the filter material and can lead to infectious respiratory diseases for the mask wearers [2].
In the following, quantification of the moisture absorption for the mouth-nose protection is investigated as a function of the material used and following the recommended wearing time for half masks in accordance with German statutory accident insurance [3]. To this end, a sample was prepared from both a cloth and an FFP2 mask. Due to the change from cloth to FFP2 masks, the structure changes from single-layer cotton fabric to multi-layer fleece. By means of thermogravimetric measurements at varying relative humidity content levels, the possible moisture absorption of the different mask types is characterized.
Measurement Conditions
For the investigations, an STA 449 F3 Jupiter® with copper furnace was coupled to the MHG 100 humidity generator. Samples of the individual mask materials (10 mm x 10 mm) were prepared from the center part (figure 1) and placed on the Pt/Ir net (figure 2) for determination of the mass changes. By means of this sample support, thermogravimetric measurements can be performed in the STA. Endo- and ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effects are not recorded. Furthermore, the specimens were aligned with the inner side of the mask facing the moisture flow, so as to mimic their real operating conditions.
The detailed measurement condtions are listed in table 1.
The temperature program was established in accordance with the investigations of the Münster University of Applied Sciences regarding reusability of FFP2 masks. The measurement program included 5 cycles of the temperature program shown in table 2.
Table 1: Measurement conditions
| Parameter | Cloth Mask | FFP2 Mask |
| Sample mass | 16.313 mg | 19.921 mg |
| Furnace | Copper | |
| Sample holder | TG sample carrier, Pt/Ir 10 net | |
| Gas atmosphere | Nitrogen | |
| Gas flow rate | 20 ml/min | |
| Accessory | MHG humidity generator | |
Table 2: Temperature program and humidity adjustment of the measurements
Measuring segments | Temperature | Rel. Humidity | Time |
1 | 32°C | 40% | 60 min |
2 | 32°C | 90% | 60 min |
3 | 32°C | 40% | 60 min |
4 | 32°C → 80°C (10 K/min) | 40% → 2.6% | - |
5 | 80°C | 2.6% | 60 min |
6 | 80°C → 32°C (10 K/min) | 2,6 % → 40 % | - |
Measurement Results
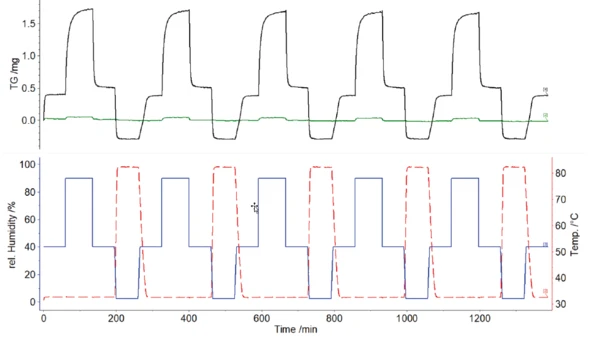
Figure 3 shows the TGA curves obtained as a function of temperature and relative humidity for the samples of both the cloth and the FFP2 masks. Both samples show a mass increase due to the increasing relative humidity, with significantly higher mass increase for the cloth sample (black) than for the FFP2 mask (green).
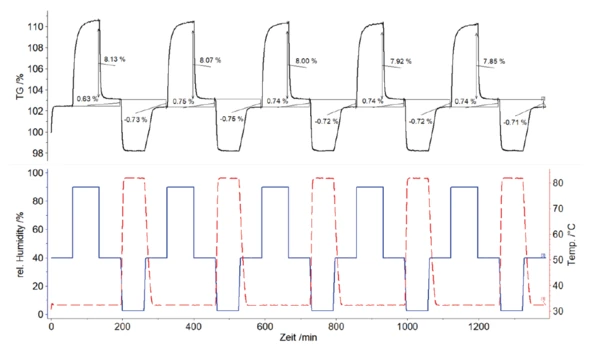
When looking at the TGA results of the cloth mask sample (figure 4) in more detail, an average mass increase of 8% can be detected following the increase in relative humidity of 40% to 90% at 32°C. This is caused by the water adsorption of the sample. When the relative humidity is subsequently reduced to 40%, a residual load of up to 0.75% remains. This absorption and desorption behavior of the cloth mask for the 5 cycles performed is reproducible and reversible.
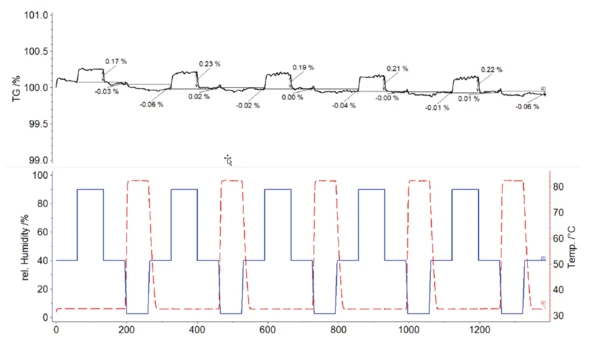
In comparison, figure 5 presents the TGA curve obtained for the FFP2 mask sample. Like the cloth mask, this material also shows a mass increase as soon as the relative humidity is increased to 80% at 32°C. However, the mass increase is significantly lower; it is only about 0.2%. Reducing the relative humidity content to 40% ensures complete release of the humidity absorbed. In contrast with the cloth mask, a residual load cannot be clearly detected for the FFP 2 mask sample. As a result, even the temperature increase to 80°C does not cause any further significant mass change.
Summary
Coupling of an STA 449 F3 Jupiter® equipped with copper furnace to a humidity generator offers the possibility of obtaining detailed insight into the mass change of a wide variety of samples as a function of variable humidity content levels. While mouth-nose protection is worn, it is continually exposed to moist respiratory air. By investigating the mass change at different moisture content levels, conclusions can be drawn about the absorption capacity or residual moisture load of the individual mask materials. The results clearly indicate that the cloth mask absorbs significantly higher amounts of moisture than the FFP2 mask and shows a residual load after the moisture content is reduced. The low load of the FFP2 mask can possibly be explained by the different layers as well as the materials used in the FFP2 mask. It is possible that the individual layers possess different properties with regard to their reaction with moisture. This characterization, however, requires further investigation.
The cloth mask sample shows a stronger moisture penetration which is only released in its entirety at elevated storage temperatures. The temperature treatment at 80°C therefore ensures complete drying of the mask and also prevents spread of bacteria and/or fungi within the cloth.