Introduction
The estimated measuring time – along with the reliability and significance of the results – often plays an important role in almost any analytical question. The more intensively analysis methods are linked to production processes, the more important this becomes. While in the research and development of new materials, measuring times for the characterization of properties are scheduled as a matter of course, in in-process analysis, it is the capacity of production plants which determines the intervals at which product properties and product quality must be verified. Analyses for quality assurance must therefore often be realized on-line during the production process, or it must at least be possible to carry them out within the space of a few minutes for random sampling control.
In the past, it had been difficult to cover these areas by means of thermal analysis since conventional analyses take from 30 minutes to several hours, depending upon the measuring program. The measuring time depends primarily on the material to be tested and/or the temperature range which must be investigated for the characteristic material properties. Decisive parameters here are also the heating and cooling rates employed. These, in turn, are essentially dependent on the constructional design of the furnaces and analytical instruments. And that is where the newly developed highspeed furnace sets new standards.
With conventional thermoanalytical instruments, heating and cooling rates from 1 K/min to 20 K/min are common while the potential range is from 0.001 K/min to 100 K/min; the new high-speed furnace, on the other hand, allows for heating rates up to 1000 K/min. A heating rate of 500 K/min already reduces the measuring time from room temperature to 1000°C to under two minutes and thus increases the sample throughput tremendously.
Concept
The new high-speed furnace does not require a stand-alone instrument but extends the well-established 400 platform by another furnace type. The platform concept allows for equipping a measuring instrument with a double-furnace hoisting device for two furnaces. The high-speed furnace can therefore be mounted on the doublehoisting device combined with other furnaces. Instead of a second furnace, an automatic sample changer (ASC) can optionally be used for the high-speed furnace. Modular flexibility and particularly the combinability of the high-speed furnace with the ASC saves a great amount of time and thus directly results in an increased sample throughput.
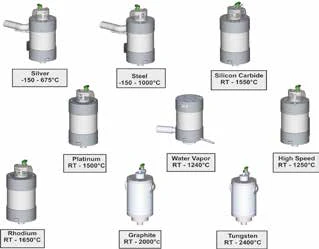
The following furnace types for the instrument series STA 449F1 and STA 449 F3 are now available.
Setup
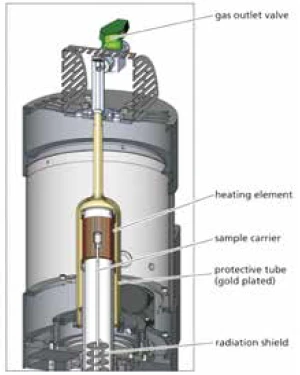
Figure 2 shows a cross section of the high-speed furnace. It can be seen that the high-speed furnace does not differ from the other furnaces of the 400 platform with regard to the main design points such as measuring heads, position of the sample temperature determination, gas flow, and separation of the sample and weighing chambers.
The great variety of crucible types and materials can also be used in the high-speed furnace. This guarantees ideal comparability of the test results, even when obtained with different furnace types.
The actual heating element of the high-speed furnace consists of a resistance-heated platinum mesh. The protective tube separates the sample chamber from the exterior and renders it possible to work in pure sample atmospheres by means of evacuating and flooding of the sample chamber.
Measurement Results
In addition to the measurements at high heating rates, measurements at conventional heating rates of 10 K/min and 20 K/min were also carried out with the high-speed furnace in order to guarantee the comparability of test results with those obtained using other thermoanalytical instruments.
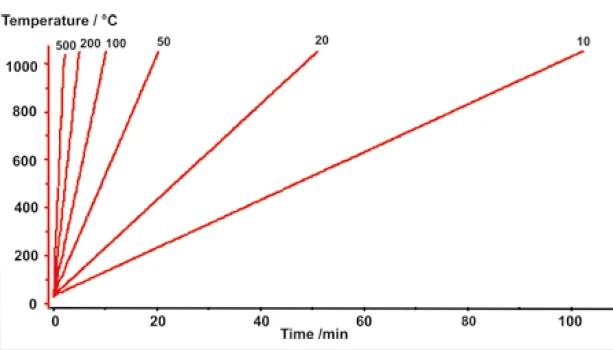
The presentation of the measured sample temperature versus time in Figure 3 shows linear heating rates in the range from 10 K/min to 500 K/min.
It was thereby confirmed that the high-speed furnace need not be limited to fast heating rates but that it is also perfectly capable of handling more conventional applications.
Varying the heating rate under otherwise identical test conditions shifts the results to higher temperatures as the heating rate increases. This is a well-known correlation which further allows for the kinetic evaluation of the measured data by means of the specially developed NETZSCH Thermokinetics software.
If the correlation between the variation in the heating rates and the effects on the measured data is known and can be mathematically described, measurements can be carried out rapidly without having to forego the traceability of the measurement data to known sample properties, as are listed in the NETZSCH annuals, for example.
Using the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of polypropylene (PP) as an example, the dependence of the results on the heating rate shall be pointed out.
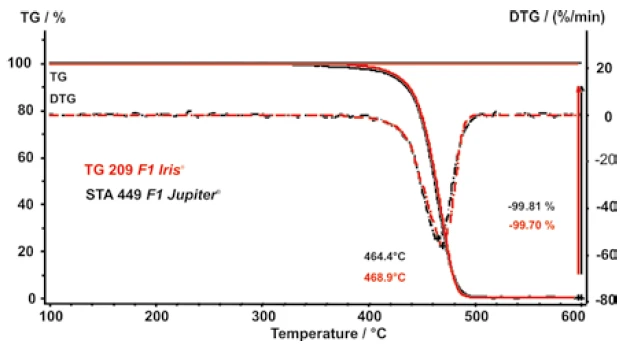
Figure 4 initially shows that there are no significant differences in the measurement results when polypropylene is investigated under identical conditions using two different thermogravimetric instruments (TG 209 F1 and STA 449 F1 ).
This is noteworthy since the furnace eometry and therefore also the flow conditions of the purge gases are different.
In addition to the results of the relative mass change (TGA), figure 4 shows its first derivative, i.e., the mass-change rates, as dashed lines (DTG). When evaluating the temperatures for the heating rates 10, 20, 50, 100, 200 and 500 K/min, where the mass-loss rate is at maximum (minimum of the DTG curve), the heating-rate dependence of the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of propylene is obtained. This is presented in figure 5.
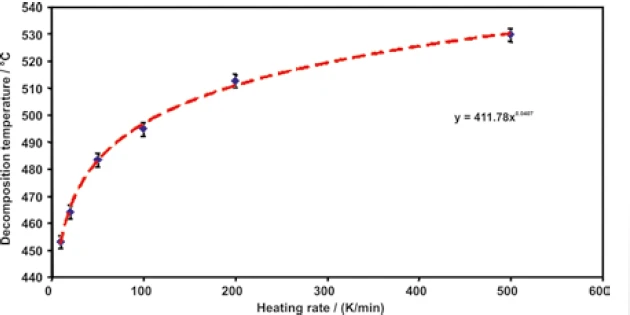
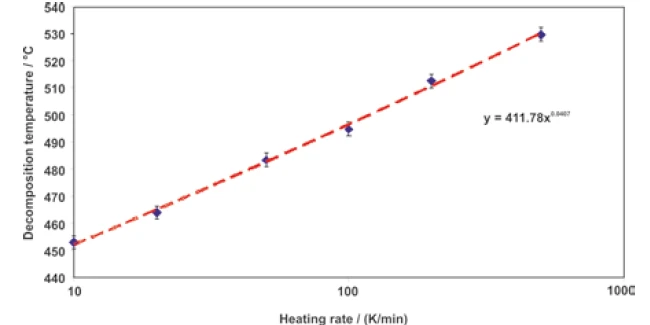
The logarithmic scaling of the heating rates yields a straight line, as can be seen in figure 6. The error bars shown in both figures 5 and 6 in the y-direction do not display real errors, but only depict a confidence interval of ± 2.5 K.
The thermal treatment of calcium carbonate (CaCO3) results in a Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction above temperatures of 600°C where calcium oxide (CaO) and carbon dioxide (CO2) are formed according to the following equation:
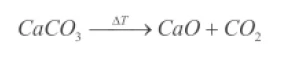
While the solid CaO remains in the sample crucible, the CO2 and the purge gas flow are both leaving the instrument via the outlet. The amount of CO2 accrued can be quantified as a mass loss.
Figure 7 presents the results of a test series which was carried out with the same measurement conditions as described for PP. The mass-loss steps are not dependent on the heating rate; the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperatures (DTG minimum) are shifted to higher temperatures as the heating rates increase.
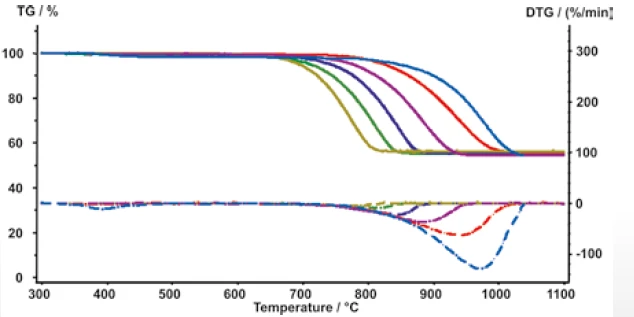
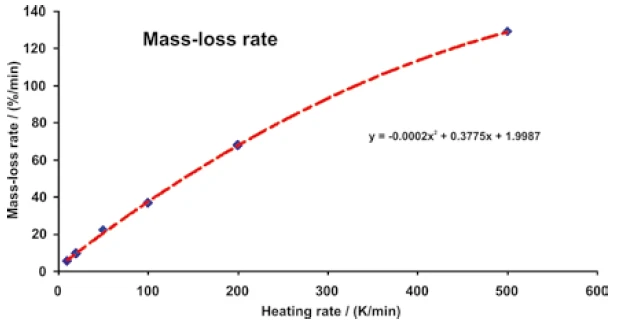
The mass-loss rate increases from 5.1%/min to 128.8%/ min when the heating rate is increased from 10 K/min to 500 K/min (Figure 8).
This shows that the influence of the heating rate on the measurement results follows a traceable law.
This relation is decisive for the comparison of measurement results which were determined at different heating rates.
Materials for products such as brake pads can now be analyzed under operating conditions. During braking, kinetic energy is transferred into heat by means of friction. The material can thereby be exposed to very high temperatures within a very short time frame.
Heating rates of 500 K/min allow these extreme operating conditions to be analytically reproduced (figure 9).
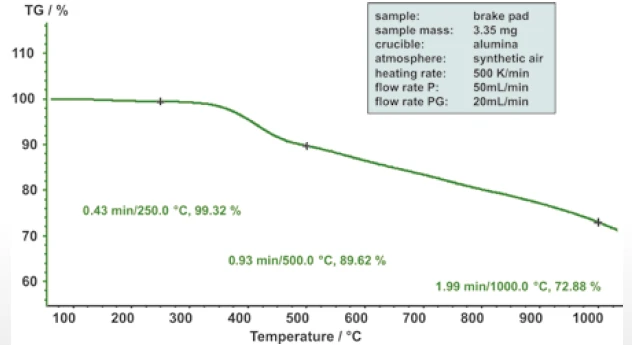
Table 1: Technical data of the high-speed furnace
Technical Data High-Speed Furnace
| Atmosphere | Inert, oxidizing |
| Sample Carrier | Standard STA |
| Max. heating rate (linear) | 1000 K/min |
| Max. sample temperature | 1250°C |
Summary
The new high-speed furnace constitutes an extension to the well-established 400 platform which enhances its already versatile potential. Some of this entails the possibility of combining the high-speed furnace with other furnaces on a double-hoist device or with an automatic sample changer (ASC). The comparability of the measurement results of the high-speed funace with those of other thermogravimetric instruments was demonstrated using the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of polyopropylene as an example. This is an important prerequisite for the unrestricted utilization of the information content of measurements at heating rates of up to 500 K/min.
The dependence of the measurement results on the variation of the heating rate shows a linear correlation under logarithmic scaling of the heating rate. Therefore, comparisons with measurements at conventional heating rates are also possible. The mass-loss steps themselves are not dependent on the variation of the heating rates.
Also, using the thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of CaCO3 as an example, it was shown clearly that although the heating rate does have an influence on the measurement results, it also follows a very traceable law.
Using fast heating rates therefore does not result in any loss of information, and the fact that each measurement only takes a few minutes yields a tremendous gain in time which greatly increases the sample throughput and thus also the efficiency of the thermoanalytical instrumentation.
The thermogravimetric investigation of a brake pad at 500 K/min also allowed – in addition to the greatly increased throughput – for materials being exposed to extreme thermal conditions to be analyzed under operating conditions for the first time.