Introduction
Zircaloy BCR-276 (Zirc-4) is a certifi ed European Commission reference material. Zircaloys are common cladding materials in thermal reactors because of their low thermal neutron absorption cross-section and their excellent thermal and mechanical properties. During the earthquake/tsumanirelated accident at the Fukushima-Daiichi nuclear plant in Japan, hydrogen accumulated under the roof of the building and was ignited. One way to produce hydrogen under these extraordinary conditions could follow the relative simple chemical reaction (1):
Zr + 2 H2O --> ZrO2 + 2 H2 + ΔH
To confirm this reaction, some preliminary experiments were carried out and are described below.
Experimental
A NETZSCH STA 449 F3 Jupiter® was equipped with a water vapor furnace and a QMS mass spectrometer QMS 403 Aeolos. Three cylinders from BCR-276 (sample weight approx. 600 mg) were placed on an alumina plate on a TG sample carrier. The samples were heated with 5 and 10 K/min under N2 and water vapor to 1050°C. The intensities of water and hydrogen were monitored with the mass spectrometer.
Results and Discussion
Figure 1 depicts the TG curve (mass change) and the intensities of hydrogen and water versus temperature for the 10 K/min measurement. After the start of the weight increase due to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation also the hydrogen level increases.
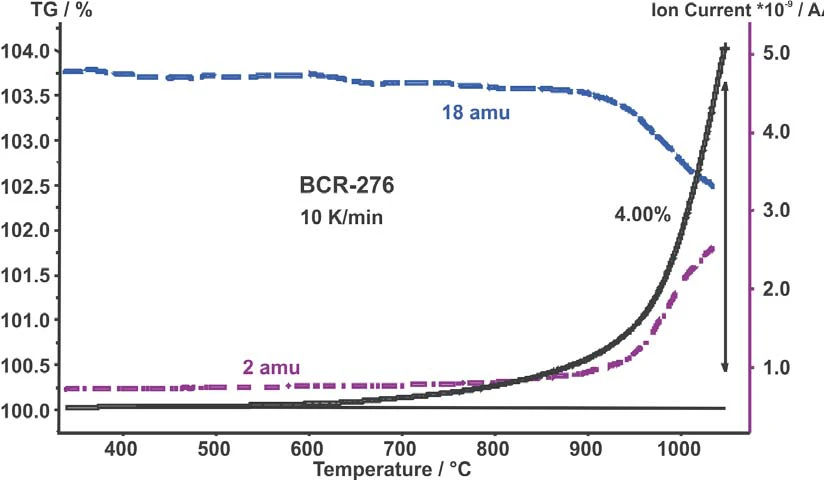
Simultaneously to the hydrogen increase, the water intensity decreases. The weight increase up to 1050°C was 4 wt%.
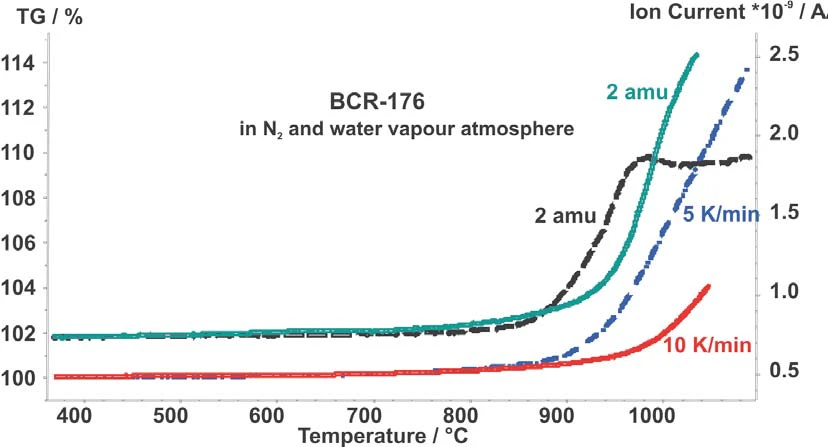
In figure 2, the TG curves and the intensities of hydrogen for the two measurements at 5 K/min and 10 K/min were compared. At a heating rate of 5 K/min the OxidationOxidation can describe different processes in the context of thermal analysis.oxidation and the hydrogen evolution starts earlier than at 10 K/min. At about 950°C, the hydrogen evolution goes into a stable saturated state (constant level).
Figure 3 shows the sample before and after the measurement.