Introduction
Along with the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, λ, the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity, a, is an important thermophysical parameter. In contrast to Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, which describes stationary heat transfer, Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity, α, is a parameter for a material's transient heat transfer. To calculate of the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity, a, is required in addition to the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity, cp , and DensityThe mass density is defined as the ratio between mass and volume. density, ρ:
λ = α·cp·ρ
The Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity is only dependent on the chemical composition. The DensityThe mass density is defined as the ratio between mass and volume. density is a function of the macroscopic structure of a material (e.g., pores). The Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity depends on the macrostructure, but also partly on the microstructure of a sample.
In the following, the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity of a copper sample is shown as a function of grain size. As a rule, the smaller the grain size (= the more grain boundaries), the lower the thermal diffusivity. The structure of a copper sample, produced by means of additive manufacturing is characterized by many small grains and thus many grain boundaries, due to the relatively short heating and fast cooling cycles. Tempering the sample (1 h at 1000°C) yields a structure with significantly larger grains and thus fewer grain boundaries. A comparison of the microstructures is depicted in figure 1.
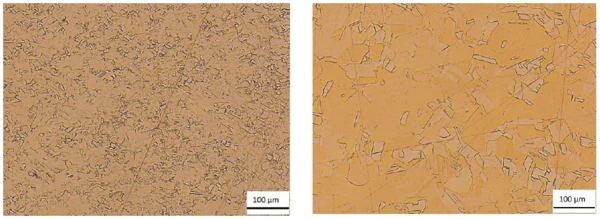
Measurement Conditions
Measurement of the thermal diffusivity at room temperature of the two copper samples was carried out with the LFA 467 HyperFlash®. The LFA samples had a diameter of 12.7 mm and a thickness of 3 mm. The samples were lightly, but not opaquely, coated with graphite prior to the measurement to improve the emission and absorption properties of the copper samples.
Measurement Results
The results are summarized in table 1. The tempered sample, at 116.88 mm²/s, exhibity nearly the literature value of pure copper, at 117 mm²/s [1]. The copper sample directly after additive manufacturing, with a smaller-grained microstructure, shows a significantly lower thermal diffusivity of 108.97 mm²/s.
Conclusion
LFA is a non-contact measuring method that can reliably resolve even small differences, such as those caused by a change in microstructure, without the disturbing influence of contact resistances.
Acknowledgement
We would like to thank Infinite Flex GmbH for the additive manufacturing and tempering of the copper samples and the University of Bayreuth, Department of Metals, for providing the micrographs.
Table 1: Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.Thermal diffusivity of pure copper with different structures at room temperature
| Sample | Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.Thermal Diffusivity/mm²/s | Deviation from the Literature Value of Pure Copper |
|---|---|---|
| Copper, directly after additive manufacturing | 108.97 | -6.8% |
| Copper, tempered (1 h @ 1000°C) | 116.88 | -0.1% |