Introduction
In the case of elastomers, the thermophysical properties below room temperature often need to be known. For example, elastomers are frequently used as seals in components or machine parts, and thus, the lower temperature limit becomes relevant. In most cases, it is of interest to understand in which temperature range an elastomer material can still reliably fulfill its function in the respective application range.
Experimental
The LFA 467 HyperFlash® can cover a temperature range of -100°C to 500°C with only one furnace. The following measurements show the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of two elastomers (NBR and NR), investigated between -100°C and 60°C. Measurements in the low-temperature range (T<0°C) require the MCT detector (Mercury-Cadmium-Telluride) and a liquid-nitrogen cooling (in this case, the NETZSCH CC300 cooling system) without having to modify the furnace. The Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity was determined by means of the DSC 204 F1 Phoenix®.
Measurement Results
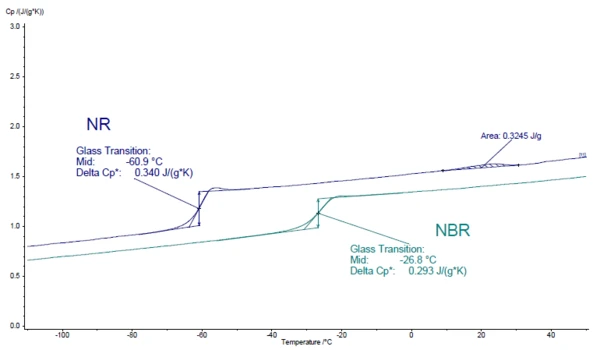
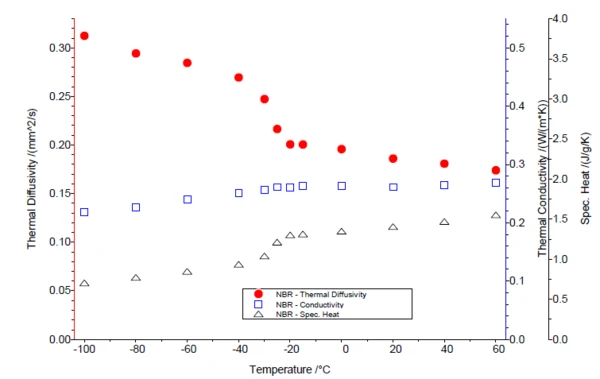
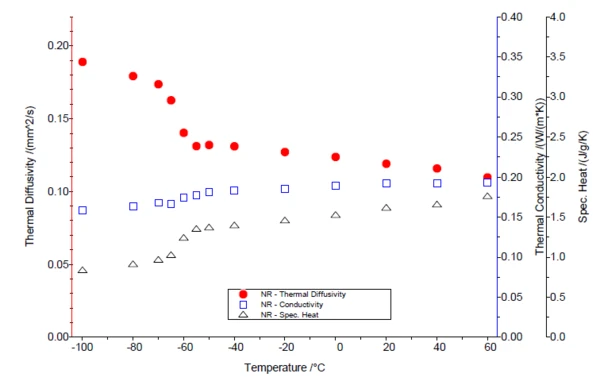
Figure 1 shows the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of the two samples. As usual for elastomers, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is below RT (NR = -60.9°C; NBR = -26.8°C) and appears as a step in the cp curve. The thermophysical properties of the two elastomer samples – the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity, Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity and the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity – are compared in figures 2 and 3. In the LFA measurement, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition can be seen through a clear decrease in Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity. The Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, on the other hand, rises almost linearly with increasing temperature and shows no significant step.