CERAMICS & GLASS
Glass Wool — Phase Transitions
Glass Wool is often used for the insulation of houses and heating pipes.
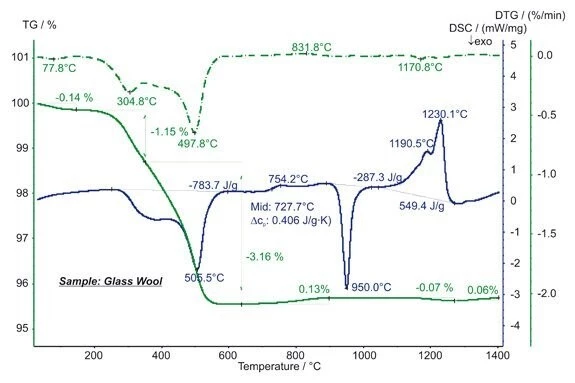
The STA measurement shows three mass-loss steps below approx. 600°C, which are due to the evaporation of humidity and the burn-up of organic binder. The latter can be seen from the strongly ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic DSC signal in this temperature range. The step in the DSC signal at 728°C with an increase in the specific heat of 0.41 J/(g*K) is due to the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition. The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic DSC peak at 950°C with an enthalpy of -287 J/g is due to CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization; the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effects between approx. 1050°C and 1250°C with an entire enthalpy of 549 J/g show the melting. The slight mass changes above 700°C are most probably due to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation and evaporation of impurities. (measurement with STA 449 F3 Jupiter®®)