Glossary
Crystallization
Crystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.
During crystal growth, artificial conditions are established under which crystallization can be accelerated.
Crystallization can occur from gaseous, liquid or solid phases. Examples are the solidification of the melt when cooled below the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point, crystallization from a saturated solution, condensation from the evaporation phase, Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition of solid substances (PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism), and the formation of crystalline products in solid-state reactions as well as crystallization of amorphous substances.
PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).Polymorphism: PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).Polymorphism is defined as the occurrence of a substance in different solid-state forms which share the same chemical composition but exhibit differences in their structure and thus in their physical properties, as well as to some extent also in their chemical ones.
Crystallization is dependent on the cooling conditions (example – fig. 1), the additives and fillers in the polymer, and the flow conditions during solidification. Subsequent stretching also changes the arrangement of the molecules and thus the material’s properties.
The crystallization or cooling curve of a DSC measurement characterizes the enthalpy course from the liquid, amorphous state into the solid, crystalline phase state.
Crystallization influences the optical, mechanical, thermal and chemical properties of the substance and its processing.

Do you have any questions?
Suitable products for your measurement
Example
Cooling Conditions
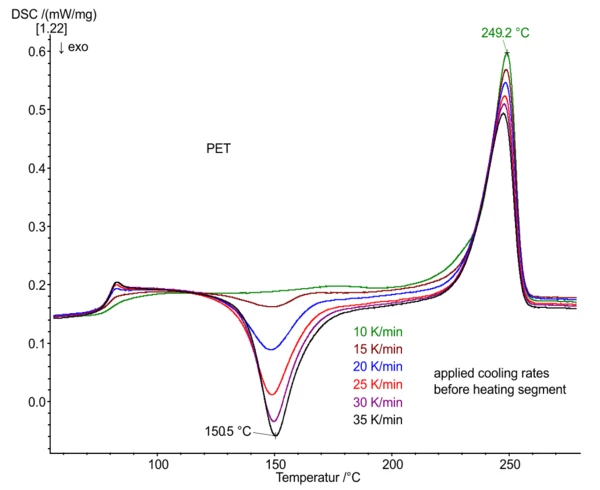
The DSC measurements show the different Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition ranges (T: 75°C to 85°C), cold crystallization (151°C) and melting effects (249°C) depending on the different cooling rates selected before heating. For defined cooling, the intracooler of the DSC 204 F1 was employed.
Polyethylene terephthalate (PET) is a semi-crystalline thermoplastic with a relatively slow crystallization rate. High cooling rates lead to large amorphous portions in this material, an increase in ∆cp at the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition and Post Crystallization (Cold Crystallization)The post crystallization of semi-crystalline plastics occurs primarily at elevated temperatures and increased molecular mobility above the glass transition.post-crystallization during heating.
Low cooling rates, in contrast, lead to the increased generation of crystalline portions that form during cooling. This results in smaller Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition steps and the absence of any Post Crystallization (Cold Crystallization)The post crystallization of semi-crystalline plastics occurs primarily at elevated temperatures and increased molecular mobility above the glass transition.post-crystallization.