16.09.2021 by Aileen Sammler, Dr. Alexander Schindler
One Click AutoEvaluation for Dilatometer Signals (dL) Improved
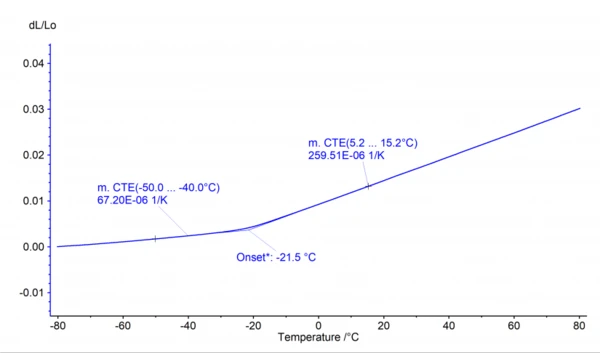
AutoEvaluation is an intelligent software algorithm and part of the NETZSCH Proteus® software, which automatically and autonomously evaluates thermo-analytical measurement curves. It has already been introduced and successfully applied for Differential Scanning Calorimetry and Thermogravimetric Analysis and is also available for dL signals as generated by dilatometers (DIL) and thermomechanical analyzers (TMA). As an improvement, the mean Coefficient of Linear Thermal Expansion (CLTE/CTE)The coefficient of linear thermal expansion (CLTE) describes the length change of a material as a function of the temperature.CTE (Coefficient of Thermal Expansion) values are evaluated additionally before and after the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition.
AutoEvaluation is an autonomous recognition and evaluation of thermal effects. The software significantly simplifies the evaluation and interpretation of thermoanalytical measurement curves. AutoEvaluation has already been introduced and successfully applied for Differential Scanning Calorimetry and Thermogravimetric Analysis. It is also available for length changes (dl/L0 signals) as generated by dilatometers (DIL) and thermomechanical analyzers (TMA).
The Objective Way to Determine Metal Melting, Glass Transition and Sintering Steps
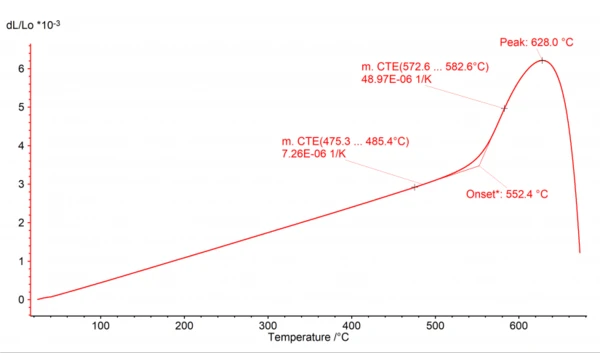
The AutoEvaluation feature of the Proteus® software for DIL and TMA consists of three independent sub-functions: “Metal Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting”, “SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering Steps” and “Glass Transition/Softening”. The “Metal Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting” function automatically evaluates the onset of the step in the dL signal occuring when a metal sample melts. The “SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering Steps” function automatically evaluates the dL steps that occur when SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering a sample. The third function, “Glass Transition/Softening”, evaluates in a self-acting, autonomous way the onset of a Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition and the maximum of dL due to softening of the sample. As an improvement, the mean Coefficient of Linear Thermal Expansion (CLTE/CTE)The coefficient of linear thermal expansion (CLTE) describes the length change of a material as a function of the temperature.CTE (Coefficient of Thermal Expansion) is evaluated additionally before and after the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition as shown in figures 1 and 2.
Adaptability of Proteus®AutoEvaluation
The three sub-functions of AutoEvaluation for DIL and TMA allow for adaption of the results to your individual application. This is also the case for DSC AutoEvaluation, which includes the “Polymer”, “Endo and Exo” and “Metal Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state.
The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting” functions. Which evaluations done by AutoEvaluation should be displayed? And which properties of these evaluations (such as peak area, onset temperature, etc.) should be shown? All this can be customized in the settings. Finally yet importantly, the user can also revise the results of AutoEvaluation.
To use the latest AutoEvaluation functions, update to the last version of the Proteus® basic software v8.03. This can be done online from the Help menu in your Proteus®® measurement or analysis software.
AutoEvaluation makes thermal analysis smarter!
Please feel free to read related blog articles and watch our videos on vimeo:
Smart Thermal Analysis (Part I): AutoEvaluation of DSC, TGA and STA curves
Smart Thermal Analyis (Part II): Identification of Measurements via Database Search
Smart Thermal Analyis (Part IIb): Identify … the Most Comprehensive Database in Thermal Analysis
Smart Thermal Analysis (Part III): AutoEvaluation of DIL and TMA curves
Smart Thermal Analysis: Measurements Wanted?