Introduction
Most cooking oils are extracted from plants or plant seeds. The first targeted cultivation of olive trees is believed to have taken place on the island of Crete around the year 3500 B.C. Besides human consumption and the production of soap, olive oil was also used in Catholic liturgy. Presented in figure 1 is an oil mill (olive crusher) in Pompeii from the cear 80 B.C.
The fruits and seeds from which oils are to be extracted are first cleaned and then crushed between rollers. Since freshly pressed oils usually also contain associated materials such as odorous-, flavoring- or bitter-substances – or plants parts, clouding agents or mucilage – they are often refined to help preserve them. To do so, the raw oil is heated; however, this leads not only to the loss of a portion of the raw oil, but also to the reduction of nutritionally and physiologically beneficial substances such as tocopherols. The polyunsaturated fatty acid content, however, is unaffected by this treatment step. Refined oils are characterized by a neutral smell and taste, a longer shelf life, and a lack of any solid sedimentaion during storage.
Cold-pressed oils are not refined but are extracted only by pressing and subsequent filtration. The heat generated during pressing is dissipated by cooling the press. The oil thus obtained is called “cold-pressed”, “cold-drawn”, “untreated“, or “unadulterated”; it is classified as very high quality [2, 3].
Fats and oils are triglycerides or triple esters of the trivalent alcohol glycerin (1, 2, 3- propanetriol). The fatty acids with which glycerin is esterified are classified as saturated, unsaturated or polyunsaturated. The reason that fats are solid at room temperature while oils are liquid is due to the unsaturated fatty acid content. Because of the increased unsaturated fatty acid content (mainly cis-positioned), CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization is hindered and the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of the oils is lowered. Therefore, a correlation is to be expected between the melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization temperatures of oils and their unsaturated fatty acid content levels.

Experimental
The melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior of commercially available cooking oils was investigated by means of the NETZSCH DSC 204 F1 Phoenix® with μ-sensor. Nitrogen was used as a purge gas (5.0); the purge gas rate amounted to 40 ml/min. The oils were introduced into standard aluminum crucibles with pierced lids in such a way as to entirely wet the bottom area of the crucibles. The measurement parameters and sample masses are summarized in tables 1 and 2.
Table 1: Measurement conditions
| Measuring Instrument | DSC 204 F1 Phoenix® |
| Sensor | μ-Sensor |
| Cooling | GN2, auto |
| Crucible | Al, pierced |
| Atmosphere | Nitrogen |
| Gas flow rate | 40 ml/min |
| Heating/cooling rate | 5 K/min |
Table 2: Sample Masses [mg]
| Olive Oil | Peanut Oil | Sesame Oil | Rapeseed Oil | Sunflower Oil | Walnut Oil | |
|---|---|---|---|---|---|---|
| Producer | A | B | C | C | D | B |
| Measurement 1 | 2.527 | 2.565 | 2.546 | 2.529 | 2.528 | 2.507 |
| Measurement 2 | 2.526 | 2.541 | 2.529 | 2.554 | 2.528 | 2.505 |
| Measurement 3 | 2.522 | 2.568 | 2.545 | 2.529 | 2.514 | 2.545 |
| Mean Value (MW) | 2.525 | 2.558 | 2.540 | 2.537 | 2.530 | 2.519 |
| Deviation (ABW) | 0.005 | 0.027 | 0.017 | 0.025 | 0.034 | 0.040 |
Results and Discussion
The cooking oils were investigated under the above-listed measurement conditions in the temperature range from -100°C to room temperature. CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization of the samples can be seen in the respective cooling segments and melting can be observed in the heating segments. Since the oils each have different content levels of saturated, monounsaturated and polyunsaturated fatty acids, and the triglycerides additionally consist of mixtures of different fatty acids, all of the samples have a relatively broad area within the melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization range in common. Figure 2 shows a comparison of the melting behavior of the various oils.
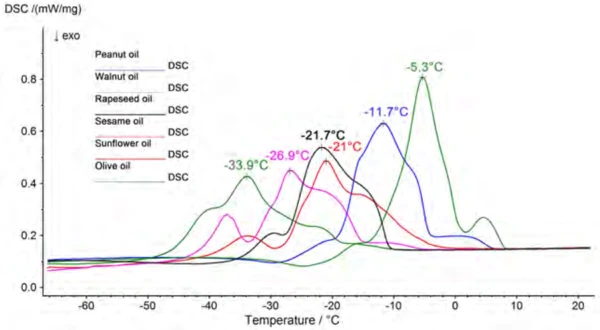
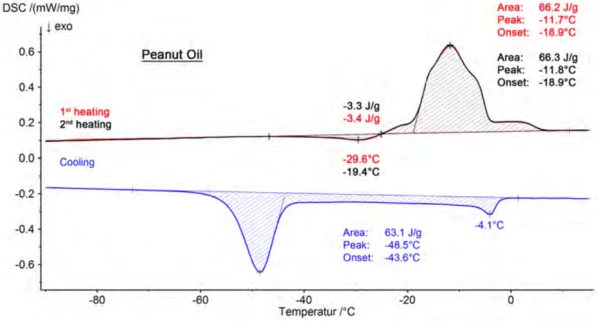
Presented in figure 3 are the results for peanut oil, consisting of two heating segments and the cooling segment in between. Beyond that, the beginning of the melting process (extrapolated onset) and the peak temperature of the main component are assessed for each sample. A comparison of these results is summarized in table 3. The values shown are mean values calculated from six measured values. A consistent trend toward lower temperatures can be seen in both the onset and the peak temperatures in the following order: olive oil, peanut oil, sesame oil, rapeseed oil, sunflower oil, walnut oil.
In comparing the content levels shown in table 4 for saturated (column 1), monounsaturated (column 2) and polyunsaturated fatty acids (column 3) in the cooking oils analyzed, in the order listed from olive oil to walnut oil, no trend can initially be seen for any of columns 1 to 3. Not even the total unsaturated fatty acid content listed in column 4 (sum of columns 2 and 3) shows a trend fitting directly to the selected sequence of the cooking oils in table 4. The relationship between the fatty acid content and the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature is more clearly illustrated in figure 4. Here, it can be seen that the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature increases as the saturated and monounsaturated fatty acid contents increase, and decreases as the polyunsaturated fatty acids increase.
Table 3: Extrapolated onset and peak temperatures of the melting processes [°C]
| Olive Oil | Peanut Oil | Sesame Oil | Rapeseed Oil | Sunflower Oil | Walnut Oil | |
|---|---|---|---|---|---|---|
| Producer | A | B | C | C | D | B |
| Extrapolated Onset | -10.1 | -18.9 | -28.3 | -28.0 | -31.5 | -44.9 |
| Peak Temperature | -5.1 | -11.8 | -21.0 | -21.4 | -26.7 | -34.0 |
Table 4: Compilation of the cooking oils and their content levels [4]
Fatty Acid Content Levels [%] | |||||
|---|---|---|---|---|---|
| saturated (S) | monounsaturated | polyunsaturated | unsaturated total (P) | P:S | |
| Walnut oil1 | 9.87 | 16.3 | 73.9 | 90.2 | 7.49 |
| Sunflower oil2 | 12.3 | 20.7 | 66.9 | 87.6 | 5.44 |
| Rapeseed oil1 | 6.9 | 57.1 | 26.9 | 84.0 | 3.90 |
| Sesame oil1 | 13.1 | 35.8 | 42.0 | 77.8 | 3.21 |
| Peanut oil2 | 16.4 | 44.8 | 38.8 | 83.6 | 2.37 |
| Olive oil1 | 15.0 | 74.7 | 9.89 | 84.6 | 0.66 |
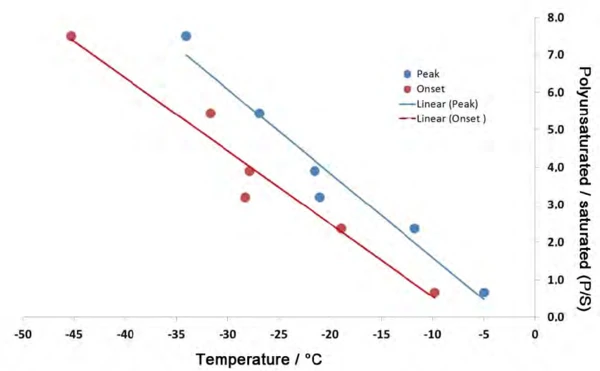
Also, in determining the quality of oils for food analysis, it is not so much the absolute values for the different fatty acid content levels as their relationships to each other that are key. Namely, if one creates a set of ratios using columns 4 and 1 – i.e., the ratio of total unsaturated fatty acid to saturated fatty acid content (P:S) – a consistent trend emerges, since the values decrease from 7.49 for the walnut oil to 0.66 for the olive oil (compare to column 5). The coloring in table 4 illustrates two groups of samples. The values marked in green describe the oils with a higher polyunsaturated than monounsaturated fatty acid content. The values marked in red, on the other hand, designate oils with a higher monounsaturated than polyunsaturated fatty acid content.
It must be taken into consideration that the information on the fatty acid content levels for the sunflower oil and peanut oil samples only reflect mean values taken from literature. Experience dictates that a fluctuation range of approximately 5% should be assumed for each value. Also, in evaluating the DSC results, only the peak temperatures of the main components were taken into consideration, which certainly only constitutes a reference point in analyzing the melting behavior of a mixture and can explain the existing deviations in the correlation diagram (figure 5). The value for the sesame oil with a P:S of 3.21 is the one which lies farthest from the trend line in figure 5. This might have something to do with the fact that this is the only oil in this set to which the seed was subjected to an additional roasting process. The influence of the roasting process onCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization is not known at this point in time.
Conclusion
This application note demontrates that the melting andCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization behavior of different cooking oils can be characterized by means of differential scanning calorimetry (DSC). Easy sample preparation and a standard temperature program allow for rapid measurement results on values for melting andCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization behavior. Evaluation based on peak temperatures make a meaningful comparison of the cooking oils simple to obtain.
Although this study fundamentally confirmed that a higher polyunsaturated fatty acid content means a lower Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature for an oil, it can also be seen in figue 4 that concentration is not the only deciding factor. Figure 5 shows that it is rather the P:S ratio – i.e., the concentration of polyunsaturated to saturated fatty acids – which exhibits a consistent trend.