
Introduction
The quality of DSC results is often determined as early as the sample preparation and measurement parameter selection phase. The crucible chosen plays an important role here. Variables such as the material, form, volume and mass of the crucible, as well as the status of the lid (yes/no/pierced/closed), are important influential factors. The first two of these – crucible material and form – will be discussed in more detail in this article.
For DSC investigations, the crucible serves primarily as a container for the sample and the reference material and – just as with a pot on a stove – must protect the sensor from contamination and distribute the heat to the sample or reference material as evenly as possible without reacting with it. Additionally, the crucible should provide good heat transfer to the sensor so that even the slightest change in the sample can be detected. Crucial factors here are the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of the crucible material and the degree of contact between the crucible bottom and sensor.
High Thermal Conducivity Provides Good Heat Transport
The Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of a material (symbol: λ) describes the transport of energy – in the form of heat – through a body based upon a temperature gradient. The higher the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity, the greater the amount of energy transported and thus the more effective the heat exchange.
The thermal conductivities of various crucible materials are summarized in table. 1. It confirms that metals have a higher λ value than, for example, ceramics (alumina) and are therefore better heat conductors. The Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of aluminum, at 237 W/(m·K), is higher than that of platinum and far higher than that of alumina, but still considerably lower than that of gold, copper and silver.
Table1: Thermophysical data of some typical crucible materials at RT
Material | Thermal conductivity λ (W/(m·K)) | (mm²/s) | (J/(g·K)) |
|---|---|---|---|
| Aluminum | 237(1) | 98.8(3) | 0.9(1) |
| Platinum | 71.6(1) | 25(3) | 0.13(1) |
| Al2O3 (α) | 28(3) | 10.2(2) | 0.76(2) |
| Copper | 404(1) | 117(3) | 0.39(1) |
| Silver | 429(1) | 173(3) | 0.23(1) |
| Gold | 317(1) | 127.2(3) | 0.13(1) |
Figure 1 illustrates the abovementioned differences by means of three different measurements on indium in aluminum, Al2O3 and platinum/rhodium crucibles. With the same sample mass and otherwise identical conditions, the measurement carried out in the aluminum crucible (red curve) exhibited the largest peak followed by the one in the Pt/Rh crucible (blue). The dotted black curve exhibits the smallest peak and represents the measurement in the Al2O3 crucible. Silver and gold create alloys when coming into contact with indium and were therefore not included in this test series.
The good heat transfer properties of the metals are reflected not only in the corresponding peak heights, but also in the so-called time constant. This is defined as the amount of time a measuring signal needs to decrease from the top of its peak to 1/e of the intensity (corresponds to a decline of approx. 63 %). Even without precise numerical data, it can be seen in figure 1 that the slope following the melting peak declines much less sharply for the measurement conducted in the Al2O3 crucible than for those conducted in the metal crucibles. The narrower a peak (e.g., the shorter the time constant), the better neighboring effects are separated, and therefore, the better the resolution. Pivotal factors here are the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity (symbol: a), which indicates how fast a material reacts to a temperature change and the thermal mass (m·cp) (for a and cp, also see table 1).
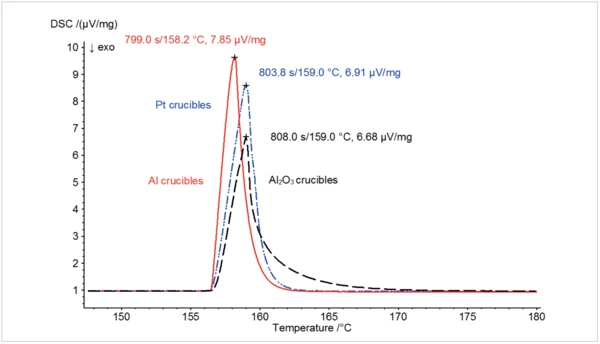
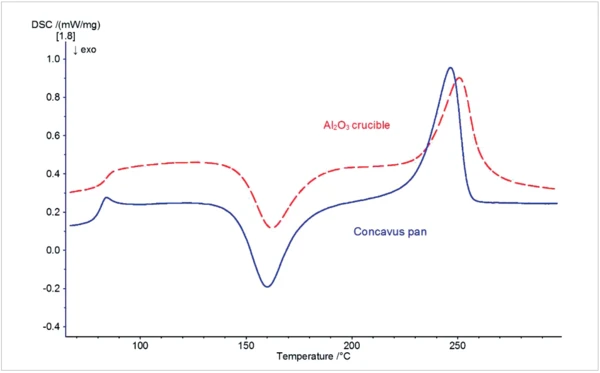
Figure 2 shows a real sample measurement on PET, carried out in aluminum crucibles (here in Concavus® crucibles, blue curve) and in Al2O3 crucibles (red dotted line). The DSC, reflecting the test in aluminum crucibles, is superior here to the measurement in Al2O3 crucibles both in terms of peak intensity (higher) and peak width (narrower).
The fact that aluminum is considerably less expensive than the precious metals gold and silver and that it also does not have a catalytic effect on organic materials, as would copper (buzz phrase: oxidative stability of cable sheathing in copper crucibles), have made aluminum to the standard crucible material for polymers, many pharmaceuticals and food. The Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of pure aluminum is 660.3°C, so the temperature range for the use of Al crucibles is limited to a maximum of 610°C.
Crucible Shape – Form Follows Function
Another factor in optimizing heat transfer is good contact between the crucible bottom and the sensor. Theoretically, a perfectly plane crucible bottom positioned onto a perfectly plane sensor would be the ideal combination. One must take into consideration, however, that even metal surfaces which are macroscopically plane consist of microscopic elevations and depressions attributable to surface roughness – so where the plane surfaces of a crucible and a sensor come together, contact is only ever actually made at certain points. The more such points there are, the better the heat transfer will be.
In addition, particularly for crucibles with a relatively thin bottom, manufacturing tolerances must not be disregarded. Even small anomalies in the plane surface of a crucible bottom can considerably reduce the reproducibility of the measurement results for such crucibles.
A new approach for meeting these challenges is to lend a concave shape to the crucible bottom, i.e., to deliberately create an inward concavity of the outer crucible bottom, as realized in the Concavus® crucible made of aluminum (figure 3). When placed upon a flat sensor, this results in an even, ring-shaped contact zone and considerably improves reproducibility.
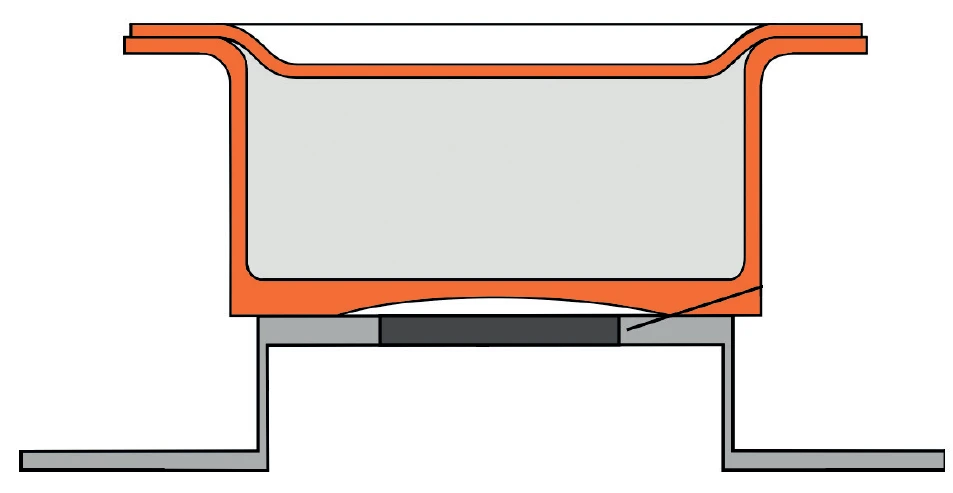
The Concavus® crucible was designed especially for the Corona sensor of the DSC 214 Polyma, but it can also be used in any other NETZSCH DSC or STA instrument with a DSC sample carrier.
At only a few millimeters high, DSC crucibles are generally quite flat. Therefore, only a small amount of heat can be lost to the surrounding gas atmosphere, and the effect on the system’s sensitivity is correspondingly positive.
Summary
Aluminum is the ideal crucible material for most measuring tasks in the temperature range to 610°C since its material and production costs are relatively low while its material properties are still very good.
The special shape of the Concavus® crucible in combination with the Corona sensor sets new standards in this realm.
As a general rule, it is important to always select crucible materials which will not interact with the sample. Whenever possible, metal crucibles should be favored for DSC investigations due to their superior heat transfer properties.