Introduction
The granulate made of aluminum manganese ore is mainly employed in the metallurgical industry. It serves as a raw material for the production of aluminum-manganese alloys. These alloys are used for various applications in the automotive, aerospace, construction and electronics industries. In some cases, aluminum manganese ore granules are also employed in the steel industry as an alloying additive for certain types of steel, to improve properties such as strength and corrosion resistance.
Measurement Conditions
The energetic effects were measured with a dynamic high-temperature differential calorimeter, NETZSCH model DSC 404 F1 Pegasus®. The top-loading system allows for measurements from room temperature to 1650°C. Depending on the application, different DSC or DTA sensors can be employed; these can be easily exchanged by the operator. For the corresponding sensors, various thermocouple types (E, K, S, and B) are available, the selection of which is dependent on the temperature range and the sensitivity required. The instrument is vacuum-tight and thus allows for measurements under a pure inert gas or oxidizing atmosphere. Heating rates of up to 50 K/min are possible. The software enables calculations of onset and peak temperature, inflection points, peak area integration and more. The measurement parameters are listed in table 1.
Table 1: Measurement parameter
| Instrument | DSC 404 F1 Pegasus® |
| Sensor/sensor type | DSC cp, type S |
| Furnace | Rhodium |
| Crucibles | Boron nitirde (BN) with pierced lid and Al2O3 disks between outer crucible bottom and sensor |
| Temperature program | RT to 1650°C |
| Heating rate | 20 K/min |
| Sample weight | 30,748 mg |
| Calibration standard | Sapphire |
Measurement Results and Discussion
For the measurement, aluminum and manganese ore (ground) were mixed at a ratio of 1:1 and heated to 1650°C at a heating rate of 20 K/min using an argon atmosphere and a BN crucible with pierced lid. Figure 1 shows the DSC signal exhibiting clearly visible energetic effects with increasing temperature.
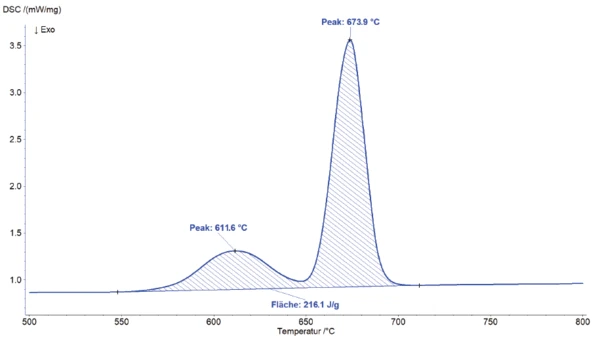
Two slightly overlapping endothermal effects are observed at the peak temperatures of 612°C and 674°C (see enlarged view in figure 2). The total enthalpy of these EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effects amounts to 216 J/g. This total effect is probably due to melting of the aluminum granulate or portion. Another EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect is detected at a peak temperature of 912°C.
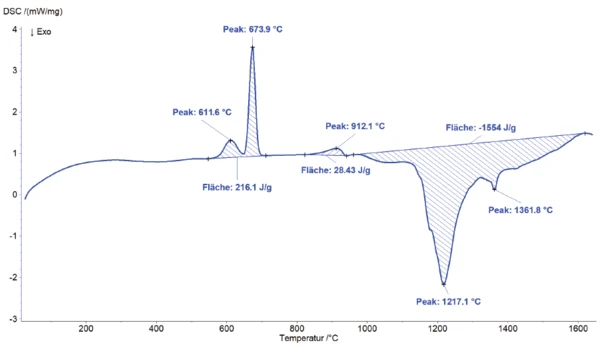
Above 1000°C, a large overlapped ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect with a total enthalpy of -1554 J/g can be seen at the peak temperatures of 1217°C and 1362°C. The overlapping, recognizable as shoulders, is most likely due to a reaction within the sample mixture. A thermite-like reaction occurs [1]. Manganese ore reacts with molten aluminum at higher temperatures by being reduced. This means that manganese reacts with aluminum, removing oxygen to form metallic manganese. The reaction takes place in accordance with the thermodynamic reactivity between the elements.
MnO2 + Al → Mn + Al2O3
The specific reaction conditions depend on the exact composition of the manganese ore and the temperature. This ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect with an enthalpy of -1554 J/g expands over a broad temperature range of over 500°C. At the end of the measurement, the sample is reweighed. A mass loss of ~ 5% is determined.
Summary
With the capability of performing thermoanalytical investigations at high temperatures, the DSC 404 F1 Pegasus® allows for analyses on materials under extreme thermal conditions. Furthermore, the imaging and characterization of large reaction enthalpies, as shown with the example above, is possible with this robust, but also very sensitive instrument.
Energetic effects and state changes can be precisely measured and analyzed, providing researchers with valuable insights into the thermal behavior and stability of a wide variety of materials over a broad temperature field.
This instrument is widely used in areas like the material and geo sciences or metal/steel and ceramics industries; i.e., in areas in which understanding and knowledge of both the thermal and thermophysical properties of materials is decisive for product development, process optimization and quality control.