Introduction
Potassium clavulanate is a salt of clavulanic acid. It is a semi-synthetic beta-lactamase inhibitor, contains a ß-lactam ring, and binds strongly to ß-lactamase at or near its active site. It helps to prevent certain bacteria from becoming resistant to the antibiotic amoxicillin. Therefore, the drug is used in conjunction with ß-lactamasesusceptible penicillins to treat infections caused by beta-lactamase-producing organisms [1, 3].
Storage of potassium clavulanate is recommended at low temperatures. Knowledge on the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition course of potassium clavulanate helps optimize the storage conditions and improves its long-time stability.
An investigation of the degradation behavior of potassium clavulanate was performed by means of the TGA-FT- IR method and is described in the following.
Measurement Conditions
The TGA measurement was carried out using the TG 209 F1 Libra® coupled to a Bruker Optics FT-IR spectrometer. For this measurement, 10.51 mg of potassium clavulanate was placed in an open alumina crucible and heated from room temperature to 600°C at a heating rate of 10 K/min under a dynamic nitrogen atmosphere (40 ml/min). In order to identify the gases released during the heating process, they were transferred directly into the gas cell of the FT-IR spectrometer via a heated Teflon transfer line.
Measurement Results
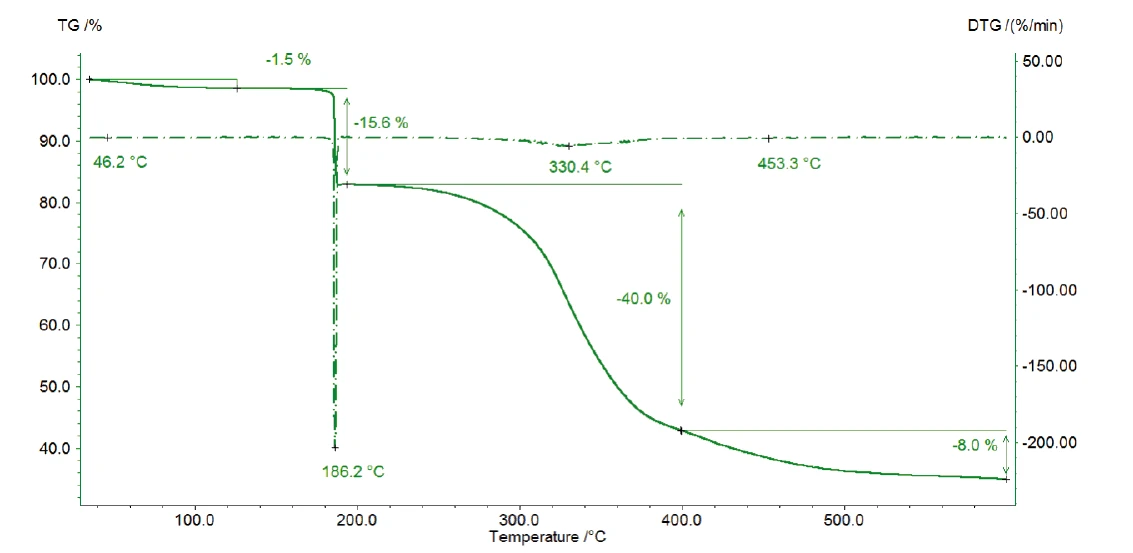
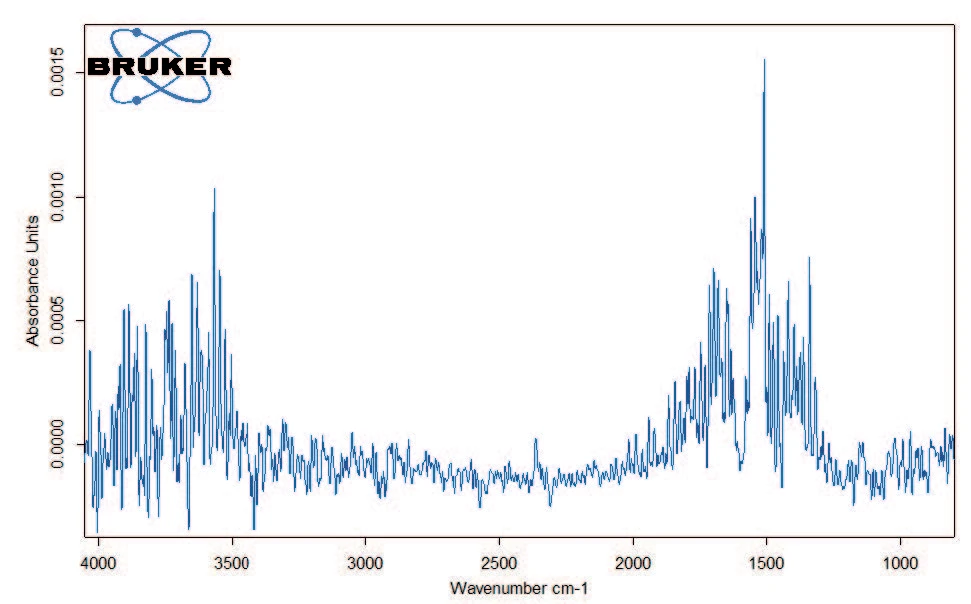
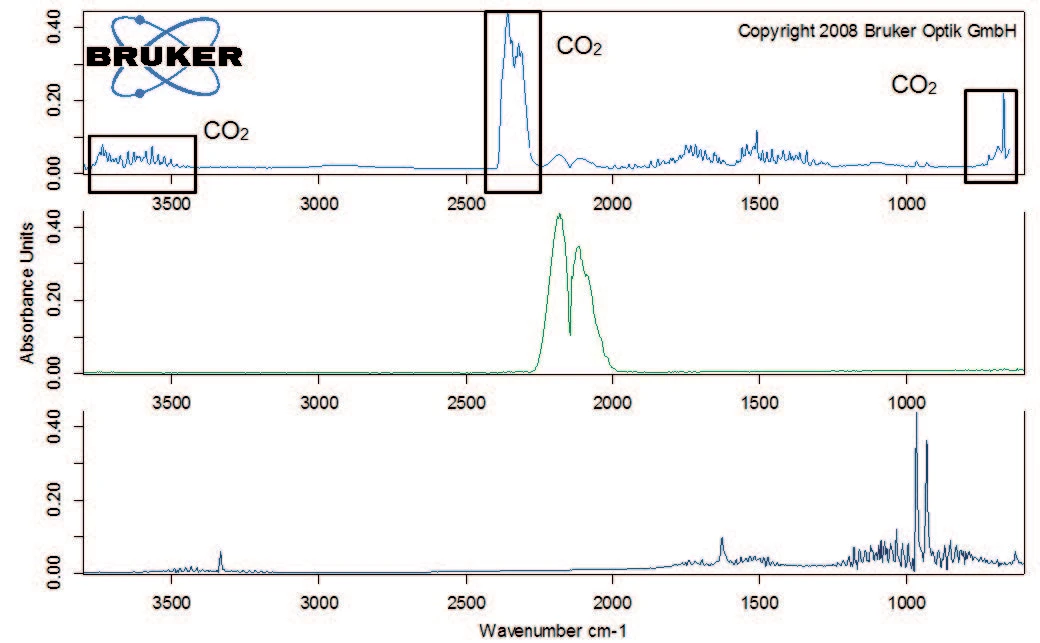
Figure 2 depicts the TGA curve in the temperature range between room temperature and 600°C. The first mass loss of 1.5% – occurring between room temperature and 110°C – results from the release of water (figure 3, FT-IR spectrum of the products released at 47°C).
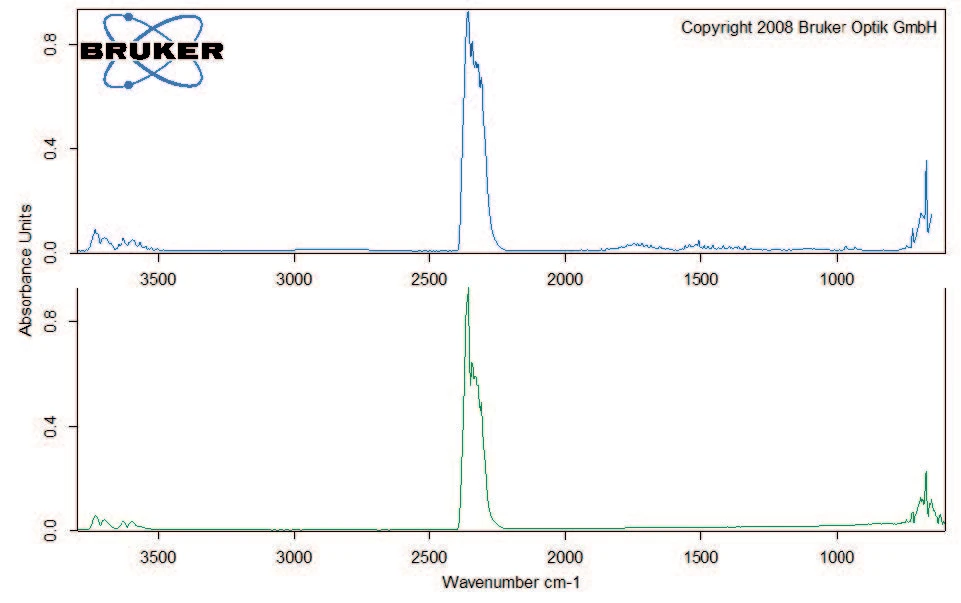
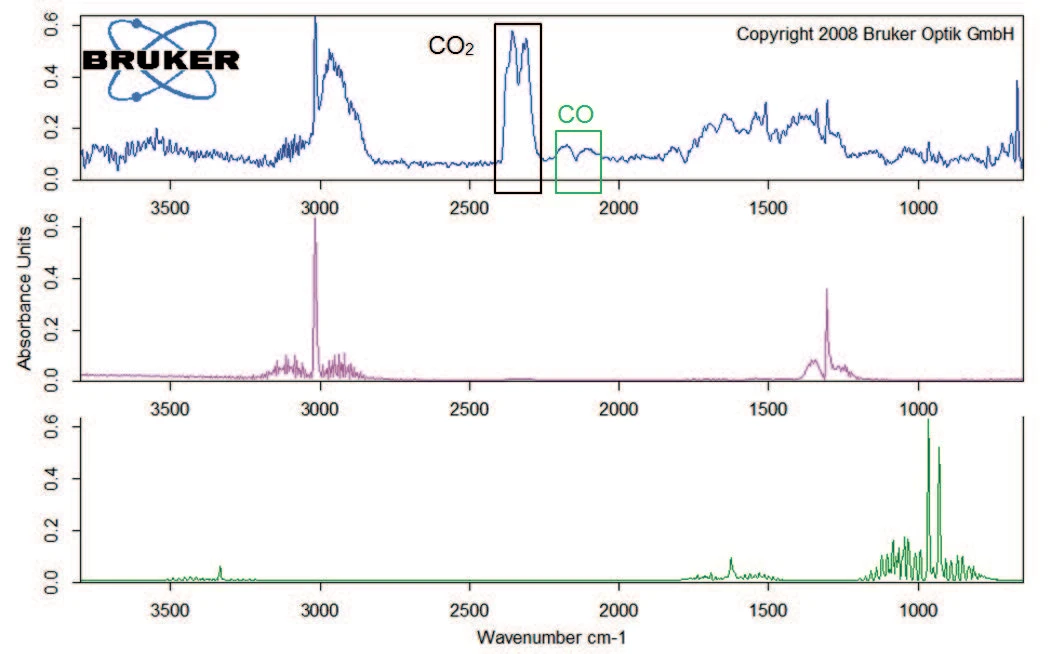
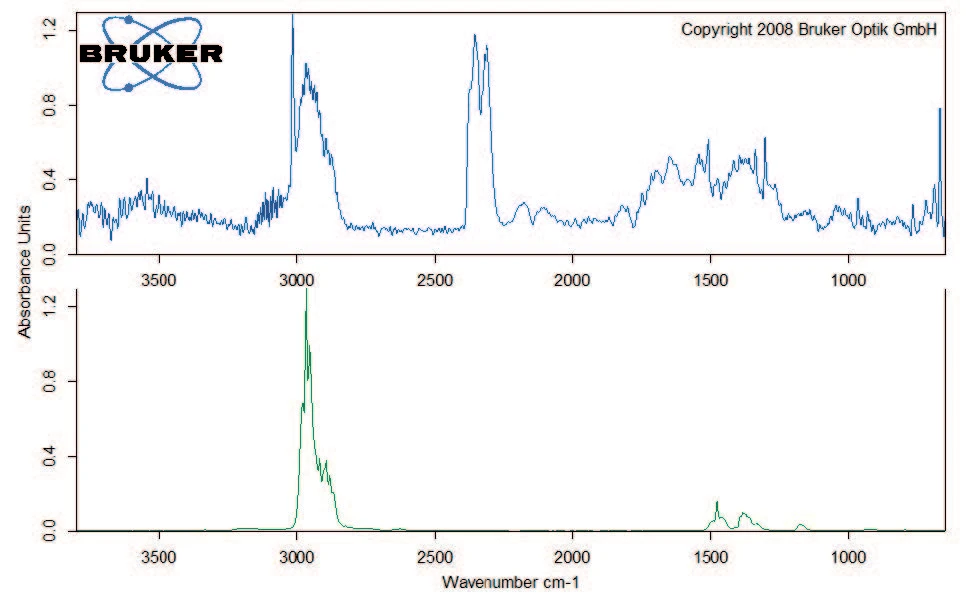
Degradation continues with a mass loss of 40% between 200°C and 400°C. Besides carbon dioxide, the gas phase includes carbon monoxide (wavenumber range from 2000 cm-1 to 2200 cm-1) and ammonia (double-band structure at approximately 950 cm-1) at 329°C (figure 5). Furthermore, the sample loses 8% in mass by the time the temperature reaches 600°C. Besides the release of carbon dioxide, carbon monoxide and ammonia, the characteristic absorptions bands for methane and isobutane can be detected in this mass-loss step (figures 6 and 7).
Conclusion
Heating potassium clavulanate to 600°C first leads to the evaporation of surface water. Thereafter, the substance degrades in different steps, first releasing carbon dioxide, and then additionally carbon monoxide and ammonia. In the last mass-loss step between 400°C and 600°C, methane and isobutane are also released.
When studying degradation by means of thermogravimetry, coupling a thermobalance to an FT-IR spectrometer is a suitable method for carrying out detailed investigations of the gases released. A heated adapter and transfer line allow for direct and fast transfer of the evolved gases into the gas cell of the FT-IR system. Such hyphenation saves measurement time by simultaneously applying two methods to the same sample under the same conditions. The recorded mass losses can simply be assigned to the gases released by means of interaction between the two software packages.