Introduction
Determination of the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of thin polymer films by means of the laser flash method is mainly limited by two factors:
- Sample thickness: Related to this are very short measurement times
- Scattered light of the flash lamp: Due to the small mass, the sample is not ideally located in the sample holder
A solution to this offers the LFA 467 HyperFlash® (see figure 1). Due to its high data acquisiton rate of 2 MHz, a short pulse time (up to 20 μs) and a special sample holder for thin samples (see figure 2), measurements on samples with a small thickness can simply and quickly be realized.

Measurement Conditions
An approximately 20 μm-thick polymer film was measured by means of the LFA 467 HyperFlash® between -40°C and 140 °C. In order to obtain an opaque sample, gold was sputtered onto the film prior to the measurement. Using graphite as a coating material is not recommended for such thin samples since it might influcence the measurement results. More information on the optimal coating of samples can be found under [1].
Measurement Results and Discussion
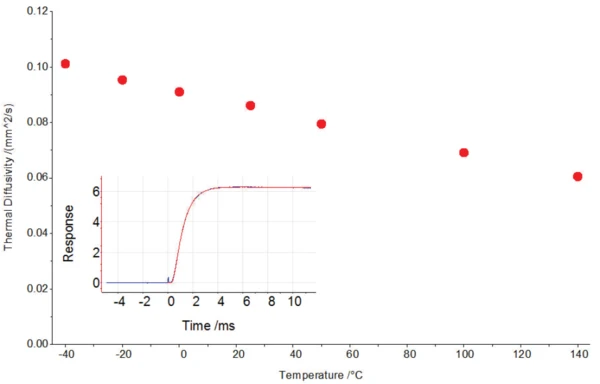
Figure 3 shows the thermal diffusitiy of the polymer film dependent upon temperature and the detector signal of a measurement. The detector signal (blue curve) can be well depicted by the mathematical model (red line). The high data acquisition of 2 MHz und a short pulse time of approx. 20 μs guarantee that very short half times (< 1 ms) can also be accurately resolved. The sample holder additionally reduces the scattered light to a minimum so that evaluation of the signal with such short half times becomes possible. For determination of the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of very thin samples, a DSC measurement is recommended. Along with the DensityThe mass density is defined as the ratio between mass and volume. density data, the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity can then also be determined.