18.08.2020 by Michael Hsu
Thermal Stability of Lithium Ion Battery Electrolyte
One of the components found in a lithium ion battery that is typically responsible for mishaps is the electrolyte. In the following article, several experiments were conducted via TGA, DSC and evolved gas analysis to investigate composition, thermal stability and identify the released products.
Lithium ion batteries are now a consistent everyday use item whether we are aware of them or not. They can be found powering our cell phones (mobile phones), laptop computers to larger items such as electric vehicles and airplanes. We often hear negative stories about mishaps that occur with them such as batteries catching on fire. One of the components found in a lithium ion battery that is typically responsible for these detrimental effects is the electrolyte.
Experimental
In the following study, several experiments were conducted via TGA, DSC and evolved gas analysis to investigate a commonly used electrolyte (1.0 M LiPF6 in EC/DEC=50/50 (v/v) acquired from Sigma-Aldrich) by subjecting it to exposure to the ambient atmosphere (N2, O2, H2O, CO2, etc.). Samples were prepared in a glove bag purged with argon utilizing approximately 8 to10 mg of electrolyte solution pipetted into 40 μl aluminum crucibles that were sealed with aluminum crucible lids that had a laser cut 50 μm pierced hole to allow gases to vent. Samples were loaded and measured with the NETZSCH STA 449 F1 Jupiter® coupled to a QMS 403 Aëolos®utilizing a 5oC/min heating rate and argon as the purge gas. Differential scanning calorimetry (DSC) was performed to monitor changes in the electrolyte’s composition. Thermogravimetric analysis (TGA) was utilized to measure thermal stability and Réaction de DécompositionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperatures, while evolved gas analysis (EGA) via mass spectrometry (MS) identified the released products.
Results and Discussion
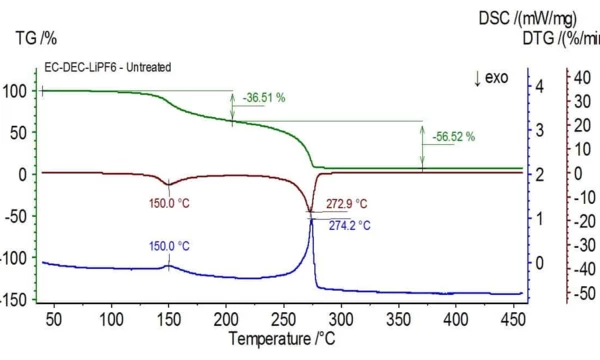
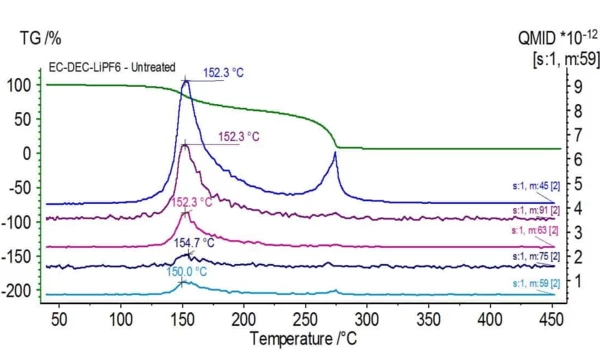
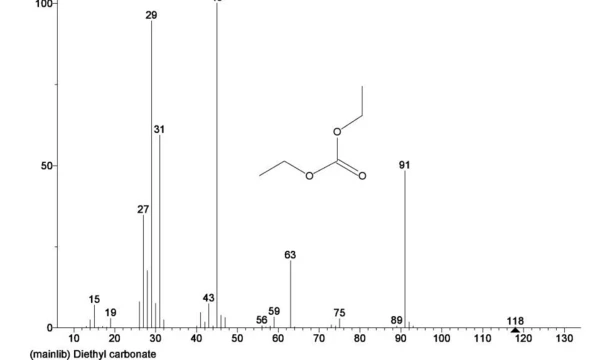
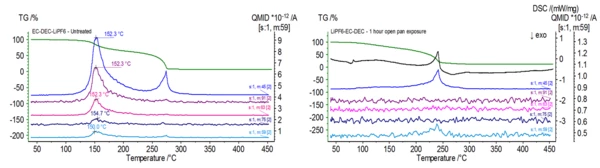
Figure 1 shows the TGA (green), DTG (brown) and DSC (blue) curves of an untreated electrolyte sample consisting of ethylene carbonate (EC), diethyl carbonate (DEC) and lithium hexafluorophosphate (LiPF6). The initial mass loss can be attributed to the evaporation of diethyl carbonate as mass numbers associated with this compound (45, 59, 63, 75 and 91) are found to be peaking around 150oC as seen in Figure 2 with the NIST library mass spectrum of diethyl carbonate shown in Figure 3.
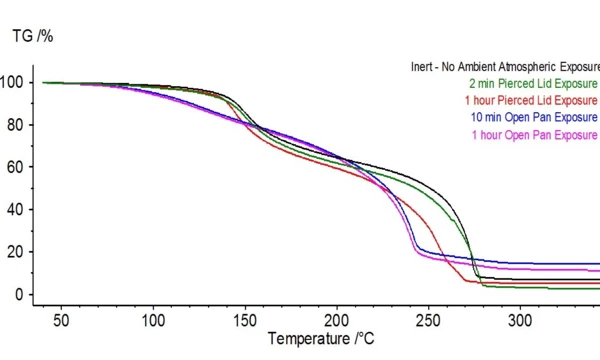
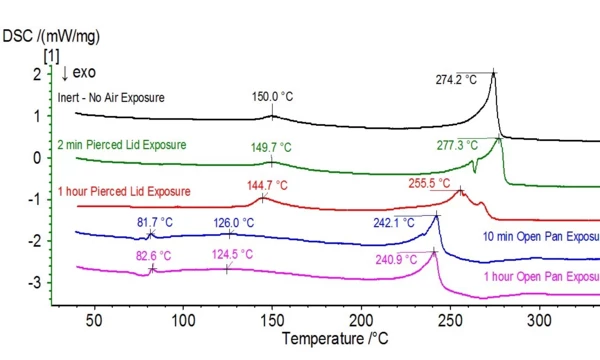
When this electrolyte is exposed to ambient atmosphere, the stability and composition begins to change. The transformation can be seen in Figures 4 and 5 where the untreated electrolyte DSC and TGA signals are plotted alongside signals of electrolyte samples that were exposed to ambient atmosphere for various durations. Evolved gas analysis (Figure 6) confirms radical changes when compared to the untreated sample as the biggest indicator was mass numbers pertaining to DEC (45, 59, 63, 75 and 91) were no longer present in the exposed sample.
Conclusion
Lithium ion battery electrolytes are known materials that are sensitive to exposure to ambient atmospheric gases. As shown, thermal and evolved gas analyzers can be used to investigate this material property that could ultimately compromise the product’s functionality and safety. The full application note is available here!